The Effect of the Polymer Type in the Three-Phases Fischer-Tropsch Synthesis Catalyzed by suspended Iron Nanocatalysts
Abstract
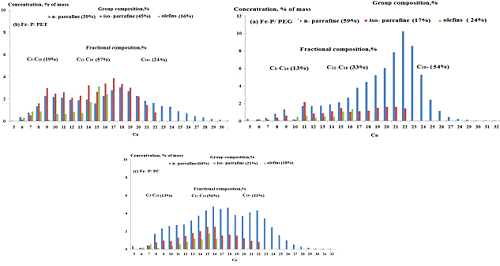
Fischer-Tropsch synthesis (FTS) was conducted over paraffin-iron catalysts of three phases system with synthetic polymers that contains different compositions. The suspended iron nanocatalyst was introduced into the slurry reactors Fischer-Tropsch with range temperature (220-320)oC at 2.0 MPa, the atomic ratio contains: 100Fe/100 Paraffin/10 wt% polymer. The study of phase, structure and morphology of the nanocatalyst using x-ray diffraction (XRD) and atomic force microscope (AFM) techniques confirmed that there are two phases of iron oxides Fe3O4 and δ-FeOOH are existed. Maximum conversion of CO to yields of total liquid hydrocarbons that obtained was 74% and 62 g/m3 of FTS over the catalyst Fe-Paraffin/ Polyethylene glycol (Fe-P/PEG) compared to Fe-Paraffin/ Polyethylene terephthalate (Fe-P/PET) and Fe-Paraffin/polycarbonate (Fe-P/PC) systems. The results shows that the polymer type and their structure as well as preparation time of the iron nanocatalysts have high influence on the particle size value. A selectivity of 65% of syngas converted C5+ liquid hydrocarbons achieved using (Fe-P/PEG) catalyst.
Full Text:
PDFReferences
- J. Blanchard, N. Abatzoglou, R. Esfandabadi, F. Gitzhofer, Fischer−Tropsch Synthesis in a Slurry Reactor Using a Nanoiron Carbide
Catalyst Produced by a Plasma Spray Technique, Ind. Eng. Chem. Res., 2010, 49 (15), 6948–6955.
- Ce. Yang, H. Zhao, Y. Hou, D. Ma, Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer–Tropsch Synthesis, J. Am. Chem. Soc., 2012, 134 (38),15814–15821.
- D. Goncalo, C. Joao, V. Bruno, G. Leticia, A. Carina, A. Maria, R. Joao, V. Pedro, Noble Metal Nanoparticles for Biosensing Applications, Sensors., 2012, 12(2), 1657-1687.
- A. Pour, M. Housaindokht, M. Irani, S. Shahric, Size-dependent studies of Fischer–Tropsch synthesis on iron-based catalyst: New kinetic model. Fuel., 2014, 116, 787-793.
- S. Ahmed, M. Ahmad, B. Swami, S. Ikram, A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. Journal of Advanced Research., 2016, 7(1), 17–28.
- Z. Wang, S. Skiles, F. Yang, Z. Yan, D. Goodman, Particle size effects in Fischer–Tropsch synthesis by cobalt. Catalysis Today., 2012, 181, 75– 81.
- S.N. Khadzhiev, N.V. Kolesnichenko, N.N. Ezhova, Slurry technology in methanol synthesis (Review). Petroleum Chemistry, 2016, 56 (2), 77–95.
- B.D. Gates, Q. Xu, M. Stewart, D. Ryan, C.G. Willson, G.M. Whitesides, New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev., 2005, 105 (4), 1171–1196.
- A. H. Al khazraji, Influence of Iron Nano Co-polymer catalysts on the liquid hydrocarbons production in the synthesis Fischer Tropsch. Journal of Global Pharma Technology. 2019, 11(07), 827-834.
- S. N. Khadzhiev, S. A. Sagitov, A. S. Lyadov, Fischer-Tropsch process in a three-phase system over iron-cobalt catalyst nanoparticles in situ synthesized in a hydrocarbon medium. Petroleum Chemistry., 2014, 54(2), 88-93.
- R. Guettel, U. Kunz, T. Turek, Reactors for Fischer‐Tropsch Synthesis. Chem. Eng. Technol., 2008, 31(5), 746-754.
- M. V. Kulikova, S.N. Khadzhiev, Metal-Containing Nano dispersions as Fischer–Tropsch Catalysts in Three-Phase Slurry Reactors. Petroleum chemistry., 2017, 57(6), 796–799.
- D. A. Grigoriev, M.N. Mikhailov.; hybrid metal - zeolite catalysts synthesis of Fischer—Tropsch for obtaining a fraction hydrocarbons с5 – с18. Catalysis in the chemical and petrochemical industries., 2013, 4, 31-41.
- M. V. Kulikova, A. H. Al Khazraji, O. S.Dement’eva, M. I. Ivantsov, V. R. Flid, S. N. Khadzhiev, Influence of dispersion medium composition on Fischer—Tropsch synthesis in three-phase system in the presence of iron-containing catalysts. Petroleum Chemistry., 2015, 55(7), 537–541.
- A. Bing, Ch. Kang, W. Cheng, W. Ye, L. Wenbin, Pyrolysis of Metal–Organic Frameworks to Fe3O4@Fe5C2 Core–Shell Nanoparticles for Fischer–Tropsch Synthesis. ACS Catal., 2016, 6(6), 3610-3618.
- A. A. Mirzaei, S. Vahid, M. Feyzi, Fischer-Tropsch Synthesis over Iron Manganese Catalysts: Effect of Preparation and Operating Conditions on Catalyst Performance. Advances in Physical Chemistry., Volume 2009, Article ID 151489, 12 pages.
- A. H. Al Khazraji, Ph. D. Thesis, iron-nanopartecles catalysts "core-shell" in the Fisher-Tropsh reaction: synthesis, structure, properties and kinetic aspects. Moscow Technological University (Institute of Fine Chemical Technologies), Moscow, Russia (2017).
- M. V. Kulikova, M. I. Ivantsov, M. N. Efimov, L. M. Zemtsov, P. A. Chernavskii, G. P. Karpacheva, S. N. Khadzhiev, formation features of composite materials containing cobalt nanoparticles active in Fischer-Tropsch synthesis. Eur. Chem. Bull., 2015, 4(4), 181-185.
- A. H. Alkhazraji, O. S. Dementyeva, Z. Pastukhova, M. V. Kulikova, V. R. Flid, Comparative study of nano iron catalysts in the presence of the crude polymer matrix and their cross link polymer in the Fischer-Trophsch synthesis. Journal of Physics: Conf. Series., 2018, 1032, 1-10, 012065.
- A. H. AlKhazraji, A. V. Krylov, M. V. Kulikova, V. R. Flid, O.Yu. Tkachenko, kinetic model for Fischer-Tropsch synthesis over nanoparticles iron catalysts with polymer matrix in a slurry reactor. Fine Chemical Technologies., 2016, 11(6), 70-77.
DOI: http://dx.doi.org/10.13171/mjc01912021046gaa
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
