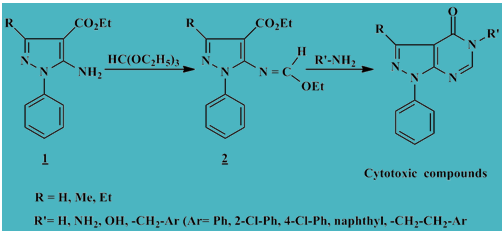
Synthesis of novel pyrazolo[3,4-d]pyrimidinone derivatives as cytotoxic inhibitors
Abstract
Full Text:
PDFReferences
- J. K. Gupta, C. Anshu, D. Rupesh, V. Kumari, P. K. Sharma, P. K. Verma, International Journal of Pharmaceutical Sciences and Research, 2010, 5, 0975-8232.
- M. H. Elnagdi, E. M. Kandeel, E. M. Zayed, Z. E. Kandeel, J. Heterocycl. Chem., 1977, 14, 155-157.
- A. E. Rashad, M. I. Hegab, R. E. Abdel-Megeid, N. Fathalla, F. M. E. Abdel-Megeid, Eur. J. Med. Chem., 2009, 44, 3285-3292.
- N. C. Desai , V.V. Joshi, K. M. Rajpara, H. V. Vaghani, H. M. Satodiya, Journal of Fluorine Chemistry, 2012, 142, 67-78.
- R. A. Mekheimer, E. A. Ahmed, K. U. Sadek, Tetrahedron, 2012, 68, 1637-1667.
- H. A. Stefani, C. M. P. Pereira, R. B. Almeida, R. C. Braga, K. P. Guzen, R. Cella, Tetrahedron Lett.,2005, 46, 6833-6837.
- A. M. Youssef, E. G. Neeland, E. B. Villanueva, M. S. White, I. M. El-Ashmawy, B. Patrick, A. Klegeris, A. S. Abd-El-Aziz, Bioorg. Med. Chem, 2010, 18, 5685-5696.
- J. P. Colomer, E.L. Moyano, Tetrahedron Lett.,2011, 52, 1561-1565.
- B. S. Holla, M. Mahalinga, M. S. Karthikeyan,P. M. Akberalib, N. S. Shetty, Bioorg. Med. Chem., 2006, 14, 2040-2047.
- M. M. El-Enany, M. M. Kamel, O. M. Khalil, H. B. El-Nassan, Eur. J. Med. Chem., 2010, 45, 5286-5291.
- D. C. Kim, Y. R. Lee, B. S. Yang, K. J. Shin, D. J. Kim, B. Y. Chung, K. H. Yoo, Eur. J. Med. Chem., 2003, 38, 525-532.
- S. Schenone, C. Brullo, O. Bruno,F. Bondavalli, L. Mosti, G. Maga,E. Crespan, F. Carraro, F. Manetti, C. Tintori, M. Botta, Eur .J. Med. Chem., 2008, 43, 2665-2676.
- M. M. Ghorab, F. A. Ragab, S. I. Alqasoumi, A. M. Alafeefy, S. A. Aboulmagd, Eur. Med. Chem., 2010, 45, 171-178.
- M. Ge, E. Cline, L. Yang, Tetrahedron Lett.,2006, 47, 5797-5799.
- K. M. Al-Taisan, H. M. Al-Hazimi, S. Al-Shihry, Molecules, 2010,15, 3932-3957.
- E. I. Al-Afleq, S. A. Abubshait, Molecules, 2001, 6, 621-638.
- P. G. Baraldi, H. El-Kashef, A. Farghaly, P. Vanelle, F. Fruttarolo, Tetrahedron, 2004, 60,5093-5098.
- A. M. F. Oliveira-Campos, A. M. Salaheldin, L. M. Rodrigues, ARKIVOC, 2007, (xvi), 92-100.
- S. Gupta, L. M. Rodrigues, A. P. Esteves, A. M. F. Oliveira-Campos, M. S. J. Nascimento, N. Nazareth, H. Cidade, M. P. Neves, E. Fernandes, M. Pinto, N. M. F. S. A. Cerqueira, N. Bras, Eur. J. Med. Chem., 2008, 43, 771-780.
- M. S. Nermien, M. M. Hany, E. S. A. E. H. Khattab, S. S. Motlaq, A. M. El-Agrody, Eur. J. Med. Chem., 2011, 46, 765-772.
- R. Yan, Y. Yang, Y. Zeng, G. Zou, J. Ethnopharmacol., 2009, 121, 451-455.
DOI: http://dx.doi.org/10.13171/mjc.2.4.2014.11.02.23
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
