Synthesis of new spiroheterocycles-fused isoxazoline from 2-arylidenes-3-phenyl-1-indanones through a regio-and diastereospecific 1,3-dipolar cycloaddition
Abstract
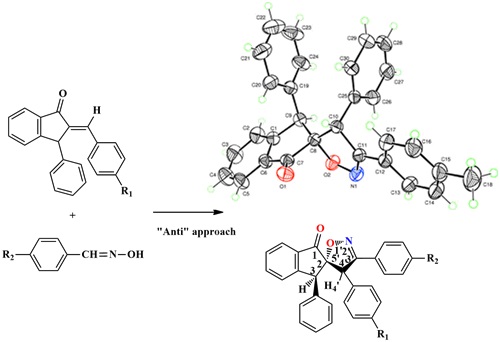
New spiroisoxazolines 3 have been synthesized by 1,3-dipolar cycloaddition of arylnitrile oxides with 2-arylidenes-3-phenyl-1-indanones. The reaction occurs in a regiospecific and diastereospecific manner and leads to one cycloadduct in all the cases. The proposed structure of the obtained cycloadducts was established based on spectroscopic data and confirmed by radiocrystallographic study. The spectral data were in favor of the observed regiochemistry and diastereoselectivity of this reaction.
Full Text:
PDFReferences
- P. Dai, X. Tan, Q. Luo, X. Yu, S. Zhang, F. Liu, W. Zhang, Synthesis of 3-Acyl-isoxazoles and Δ2-Isoxazolines from Methyl Ketones, Alkynes or Alkenes, and tert-Butyl Nitrite via a Csp3–H Radical Functionalization/Cycloaddition Cascade, Org. Lett., 2019, 21 (13), 5096-5100.
- M. Miglianico, M. Eldering, H. Slater, N. Ferguson, P. Ambrose, R. S. Lees, K. M. J. Koolen, K. Pruzinova, M. Jancarova, P. Volf, C. J. M. Koenraadt, H. Duerr, Gr. Trevitt, B. Yang, A. K. Chatterjee, J. Wisler, A. Sturm, T. Bousema, R. W. Sauerwein, P. G. Schultz, M. S. Tremblay, K. J. Dechering, Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases, Proc. Natl. Acad. Sci USA, 2018, 115 (29), E6920–E6926.
- M. Li, B. Song, M. Imerhasan, H. Youji, Progress in synthesis and bioactivity of spiroisoxazoline compounds, Chinese journal of organic chemistry, 2018, 38 (2), 378-400.
- R. Sakly, H. Edziri, M. Askri, M. Knorr, K. Louven, C. Strohmann, M. Mastouri, Synthesis of New Spirooxindole-Fused Isoxazoline/Triazole and Isoxazoline/Isoxazole Derivatives from Three-Component 1,3-Dipolar Cycloaddition, Journal of Heterocyclic Chemistry, 2017, 54 (6), 3554-3564.
- H. Deng, B. Zhang, Y. Xu, Y. Zhang, J. Huo, L. Zhang, G. Chang, A simple approach to prepare isoxazoline-based porous polymer for the highly effective adsorption of 2,4,6-trinitrotoluene (TNT): Catalyst-free click polymerization between an in situ generated nitrile oxide with polybutadiene, Chemical Engineering Journal, 2020, 393, 124674.
- C. C. Bernal, L. C. Vesga, S. C. Mendez-Sánchez, Synthesis and anticancer activity of new tetrahydroquinoline hybrid derivatives tethered to isoxazoline moiety, Med. Chem. Res., 2020, 29, 675–689.
- V. Palmieri, W. J. Dodds, J. Morgan, E. Carney, H. A. Fritsche, J. Jeffrey, R. Bullock, J. P. Kimball, Survey of canine use and safety of isoxazoline parasiticides, Vet. Med. Sci., 2020, 6 (4), 933-945.
- N. Lamassiaude, B. Toubate, C. Neveu, P. Charnet, C. Dupuy, F. Debierre-Grockiego, I. Dimier-Poisson, C. L. Charvet, Distinct molecular targets for macrocyclic lactone and isoxazoline insecticides in the human louse: new prospects for the treatment of pediculosis, bioRxiv, 2020.
doi: 10.1101/2020.08.06.239400.
- H. Abolhasani, S. Dastmalchi, M. Hamzeh-Mivehroud, B. Daraei, A. Zarghi, Derivatives as Selective Design, Synthesis and Biological Evaluation of New Tricyclic Spiroisoxazoline COX-2 Inhibitors and Study of Their COX-2 Binding Modes via Docking Studies, Medicinal Chemistry Research, 2016, 25 (5), 858–869.
- S. K. Prajapti, S. Shrivastava, U. Bihade, A. K. Gupta, V. G. M. Naidu, U. C. Banerjee, B. N. Babu, Synthesis and Biological Evaluation of Novel Δ2-Isoxazoline Fused Cyclopentane Derivatives as Potential Antimicrobial and Anticancer Agents, Med.Chem.Comm., 2015, 6 (5), 839–845.
- K. Kaur, V. Kumar, A. K. Sharma, G. K. Gupta, Isoxazoline Containing Natural Products as Anticancer Agents, European journal of medicinal chemistry, 2014, 77, 121–133.
- G. Suresh, R. Venkata Nadh, N. Srinivasu, K. Kaushal, Novel Coumarin Isoxazoline Derivatives: Synthesis and Study of Antibacterial Activities, Synthetic Communications, 2016,46 (24), 1972–1980.
- P. Das, M. H. Hasan, D. Mitra, R. Bollavarapu, E. J. Valente, R. Tandon, D. Raucher, A. T. Hammell, Design, Synthesis, and Preliminary Studies of Spiro-isoxazoline-peroxides against Human Cytomegalovirus and Glioblastoma, J. Org. Chem., 2019, 84 (11), 6992-7006.
- P. Das, A. O. Omollo, L. J. Sitole, E. McClendon, E. J. Valente, D. Raucher,L. R. Walker, A. T. Hammell, Synthesis and Investigation of Novel Spiro-isoxazolines as Anti-Cancer Agents, Tetrahedron Lett., 2015, 56 (14), 1794–1797.
- S. Pratap, F. Naaz, S. Reddy, K. K. Jha, K. Sharma, D. Sahal, Anti‐proliferative and anti‐malarial activities of spiroisoxazoline analogues of artemisinin, Arch. Pharm., 2019, 352 (1), 1800192.
- G. Al Houari, A. Kerbal, B. Bennani, M. Filali. Baba, M. Daoudi, T. Ben Hadda, Drug design of new antitubercular agents:1,3-dipolar cycloaddition reaction of para-substituted-benzadoximes and 3-para-methoxy-benzyliden-isochroman-4-ones, ARKIVOC, 2008 (xii), 42-50.
- M. Li, B. Song, I. Mukhtar, Progress in Synthesis and Bioactivity of Spiroisoxazoline Compounds, Chinese Journal of Organic Chemistry, 2018, 38 (2), 378-400.
- A. Abolhasani, F. Heidari, S. Noori, S. Mousavi, H. Abolhasani, Cytotoxicity Evaluation of Dimethoxy and TrimethoxyIndanonic Spiroisoxazolines Against Cancerous Liver Cells, Current Chemical Biology, 2020, 14 (38). doi: 10.2174/2212796813666190926112807.
- N. Najim, Y. Bathich, M. Zain, A. S. Hamzah, Z. Shaameri, Evaluation of the Bioactivity of Novel Spiroisoxazoline Type Compounds against Normal and Cancer Cell Lines, Molecules, 2010, 15 (12), 9340–9353.
- T. Ben Hadda, A. Kerbal, B. Bennani, G. Al Houari, M. Daoudi, A. C. L. Leite, V. H. Masand, R. D. Jawarkar, Z. Charrouf, Molecular Drug Design, Synthesis and Pharmacophore Site Identification of Spiroheterocyclic Compounds: Trypanosoma Crusi Inhibiting Studies, Medicinal Chemistry Research, 2013, 22 (1), 57–69.
- B. Bennani, A. Kerbal, N. Ben Larbi, T. Ben-Hadda, Moroccan patent, 2005, OMPIC, N° 27361.
- B. Bennani, A. Kerbal, N. Ben Larbi, T. Ben-Hadda, Moroccan patent, 2005, OMPIC, N° 27362.
- M. Zaki, A. Oukhrib, M. Akssira, S. Berteina-Raboin, Synthesis of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of tomentosin, RSC Adv., 2017, 7, 6523-6529.
- G. P. Savage, Spiro Isoxazolines via Nitrile Oxide 1,3-Dipolar Cycloaddition Reactions, Current Organic Chemistry, 2010, 14 (14),
–1499.
- B. Mohon Chaki, K. Wakita, S. Takizawa, K. Takenaka, H. Sasai, Enantioselective Synthesis of Spiro (Isoxazole-Isoxazoline) Hybrid Ligand, heterocycles, 2018, 97 (1), 493-505.
- T. Shimizu, Y. Hayashi, H. Shibafuchi, K. Teramura, A Convenient Preparative Method of Nitrile Oxides by the Dehydration of Primary Nitro Compounds with Ethyl Chloroformate or Benzenesulfonyl Chloride in the Presence of Triethylamine, Bull. Chem. Soc. Japan, 1986, 59, 2827- 2831.
- K. Liu, B. R. Shelton, R. K. Howe, A particularly convenient preparation of benzohydroximinoyl chlorides (nitrile oxide precursors), J. Org. Chem., 1980, 45, 3916 – 3918.
- N. Naji, M. Soufiaoui, P. Moreau, Synthèse d'isoxazolines et d'isoxazoles F-alkylés en milieu biphasique CHCl3-NaOCl, Journal of Fluorine Chemistry, 1996, 79 (2), 179-183.
- S. Lafquih Titouani, M. Soufiaoui, L. Toupet, R. Carrié, Reaction de Diels Alder de l’isoprene et du dimethyl-2,3 butadiene avec quelques Arylidene-2 Indanones, Tetrahedron, 1990, 11, 3869–3878.
- C. F. Koelsch, H. Hochmann, C. T. Le Claire, The Friedel-Crafts Reaction with Cinnamic, Crotonic, and β-Chlorocrotonic Acids, Journal of the American Chemical Society, 1943, 65 (1), 59–60.
- A. Mahfoud, G. Al Houari, M. El Yazidi, M. Saadi, L. El Ammari, Crystal Structure of 3,4’-diphenyl-3’-p-tolyl-4’H-spiro[Indan-2,5’-[1,2]oxazol]-1-one, Acta Crystallographica, Section E, 2015, 71 (11), o873-o874.
- M. Bakhouch, M. El Yazidi, G. Al Houari, M. Saadi, L. El Ammari, 3′,4′-Diphenyl-3H,4′H-Spiro[Benzo[b]Thiophene-2,5′-Isoxazol]-3-one, IUCrData, 2018, 3 (7), x181019.
- M. Bakhouch, M. El Yazidi, G. Al Houari, M. Saadi, L. El Ammari, 3′-(4-Chlorophenyl)-4′-Phenyl-3H ,4′ H -spiro[benzo[b]thiophene-2,5′-isoxazol]-3-one, IUCrData, 2017, 3 (5), x170677.
- N. Mishriky, F. M. Asaad, Y. A. Ibrahim, A. S. Girgis, 1,3-Dipolar Cycloaddition of Arylnitrile Oxides to Some Exocyclic Alkenes and Regioselective Synthesis of Spiro Isoxazolines, Journal of Chemical Research, Synopses, 1997, 12, 438.
- H. Yazdani, A. Bazgir, Lewis Acid-Catalyzed Regio- and Diastereoselective Synthesis of Spiroisoxazolines via One-Pot Sequential Knoevenagel Condensation/1,3-Dipolar Cycloaddition Reaction, Synthesis, 2019, 51 (7), 1669-1679.
- X. Li, X. Yu, Y. Feng, Synthesis of novel bis-spiroisoxazolines through 1,3-dipolar cycloaddition of nitrile oxide with - bis(arylmethylidene)cycloalkanone, Chinese Journal of Chemistry, 2009, 27 (8), 1531-1536.
- S. Boudriga, M. Askri, R. Gharbi, M. Rammah, K. Ciamala, 1,3-Dipolar cycloadditions of arylcarbonitrile oxides and diarylnitrilimines with some 2-arylmethylene 1,3-indanediones; regiochemistry of the reactions, Journal of Chemical Research, Synopses, 2003, 4, 204-207.
- R. Chekti, M. Soufiaoui, L. Toupet, Régiochimie et diastéréochimie de l’addition des arylnitrile oxides sur les énones derivées d’indanones, Bulletin des Sociétés Chimiques Belge, 1991, 100 (2), 153–157.
- N. G. Argyropoulos, E. Coutouli-Argyropoulou, 1,3-Dipolar Cycloaddition Reactions of 2-Phenyl-4-Arylidene-5(4H)-Oxazolones with Nitrile Oxides, Journal of Heterocyclic Chemistry, 1984, 21 (5), 1397–1400.
- C. Grundmann, R. Richter, Nitrile Oxides. IX. Basic Substituted Stable Nitrile Oxides, The Journal of Organic Chemistry, 1967, 32 (7), 2308–2312.
DOI: http://dx.doi.org/10.13171/mjc02105141076gh
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
