Synthesis of a new serie of quinoline-carboxamides based on methylated aminoesters: NMR characterization and antimicrobial activity
Abstract
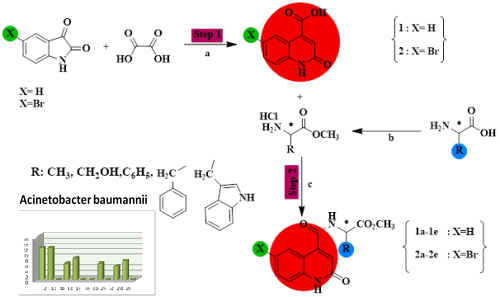
Ten new quinoline-carboxamides have been synthesized using the coupling reaction between 2-oxo-1,2-dihydroquinoline-4-carboxylic acid as a substrate and five different amino ester at room temperature with basic media (triethylamine). The products were obtained with a good yield ranging from 60 to 80 % and were structurally characterized by 1H and 13C NMR spectroscopy and mass spectrometry. The antibacterial activities of the synthesized compounds have been evaluated against 9 strains of bacteria and compared to references (erythromycin, ofloxacin, ticarcillin, oxacillin, ampicillin, norfloxacin, ceftazidim, cefotaxim). The results showed that the majority of carboxamides-quinoline ester groups present a larger inhibition diameters than those of the antibiotics references. The highest antibacterial activity in vitro against the Enterococcus feacalis has been revealed for compound 1a (methyl 2-oxo-1.2-dihydroquinoline-4-yl-L-alaninate).
Full Text:
PDFReferences
- E.A. Ashley, M. Dhorda, R.M. Fairhurst, Ali R. Keramati, M.D. Mohsen Fathzadeh, A. Mani, Spread of Artemisinin Resistance in Plasmodium falciparum malaria, N. Engl. J. Med., 2014, 371, 411-423.
- G. Y. Lesher, E. J. Froelich, M. D. Gruett, J. H. Bailey and R. P. Brundage, “1,8-Naphthyridine Derivatives: A New Class of Chemotherapeutic Agents”, J. Med. Pharm. Chem., 1962, 5, 1063-1065.
- A. S. Wagman, M. P. Wentland, Quinolone antibacterial agents, J. Compreh. Med. Chem. II., 2007, 7, 567-596.
- S. Emami, A. Shafiee and A. Foroumadi, Quinolones: Recent Structural and Clinical Developments, Iran. J. Pharm. Res., 2005, 3, 123-136.
- RF. Grossman, P-R. Hsueh, SH. Gillespie, F. Blasi, Community-acquired pneumonia and tuberculosis: differential diagnosis and the use of fluoroquinolones, Int. J. Infect. Dis., 2014, 18, 14-21.
- N. C. Desai, B. Y. Patel & B. P. Dave. Synthesis and antimicrobial activity of novel quinoline derivatives bearing pyrazoline and pyridine analogues, J. Med. Chem. Res., 2016, 26, 109-119.
- G. R. Newcome et W.W. Paudler, Contemporary Heterocyclic Chemistry, John Wiley and Sons; New York, 1982.
- F. Sliman, M. Blairvacq, Durieu, E. Meijer, L. Rodrigo, J. Desmaële, D. Bioorg, Identification and structure-activity relationship of 8-hydroxy-quinoline-7-carboxylic acid derivatives as inhibitors of Pim-1 kinase, Med. Chem. Lett., 2010, 20, 2801.
- G. Roma, G. Grossi, M. Di Braccio, D. Piras, V. Ballabeni, M. Tognolini, S. Bertoni, E. Barocelli, 1,8-Naphthyridines VII. New substituted 5-amino[1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides and their isosteric analogues, exhibiting notable anti-inflammatory and/or analgesic activities, but no acute gastrolesivity, Eur. J. Med. Chem., 2008, 43, 1665.
- G. Venkat Reddy, S. Ravi Kanth, D. Maitraie, B. Narsaiah, P. Shanthan Rao, K. Hara Kishore, U.S.N. Murthy, B. Ravi, B. Ashok Kumar, T. Parthasarathy, Design, synthesis, structure-activity relationship and antibacterial activity series of novel imidazo fused quinolone carboxamides, Eur. J. Med. Chem., 2009, 44, 1570.
- I. Tomassoli, L. Ismaili, M. Pudlo, C. de los Ríos, E. Soriano, I. Colmena, L. Gandía, L. Rivas, A. Samadi, J. Marco-Contelles, B. Refouvelet, synthesis, biological assessment and molecular modeling of new dihydroquinoline-3-carboxamides and dihydroquinoline-3-carbohydrazide derivatives as cholinesterase inhibitors, and Ca channel antagonists, Eur. J. Med. Chem., 2011, 46, 1-10.
- K. Tsuji, G. Spears, K. Nakamura, T. Tojo, N. Seki, A. Sugiyama, M. Matsuo. Quinoline-3-carbothioamides and related compounds as novel immunomodulating agents. Bioorg. Med. Chem. Lett., 2002, 12, 2427-30.
- P. Hochegger, J. Faist, W. Seebacher, R. Saf, P. Mäser, M. Kaiser, R. Weis. Synthesis and structure-activity relationships for new 6-fluoroquinoline derivatives with antiplasmodial activity, Med. Chem. Lett., 2019, 27, 2052-2065.
- A. Trivedi, D. Dodiya, J. Surani, S. Jarsania, H. Mathukiya, N. Ravat, V. Shah, Facile one-pot synthesis and antimycobacterial evaluation of pyrazolo[3,4d] pyrimidines, Arch. Pharm., 2008, 341, 435-439.
- N. Muruganantham, R. Sivakumar, N. Anbalagan, V. Gunasekaran, J.T. Leonard, Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives, Biol. Pharm. Bull., 2004, 27, 1683-1687.
- B. Chakraborty, D. Dutta, S. Mukherjee, S. Das, N.C. Maiti, Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29), Eur. J. Med. Chem., 2015, 102, 93-105.
- X. Bu, J. Chen, LW. Deady, C. L. Smith, B. C. Baguley, D. Greenhalgh, S. Yang, WA. Denny. Bioorg, Synthesis and cytotoxic activity of N-[(alkylamino)alkyl]carboxamide derivatives of 7-oxo-7H-benz[de]anthracene, 7-oxo-7H-naphtho[1,2,3-de]quinoline, and 7-oxo-7H-benzo[e]perimidine, Med. Chem. Lett., 2005, 13, 3657.
- C. A. Townsend, A. M. Brown, Nocardicin A: biosynthetic experiments with amino acid precursors, J. Am. Chem. Soc., 1983, 105, 913-918.
- M. G. Moloney, Excitatory amino acids, Nat. Prod. Rep., 1999, 16, 485–498
- A. B. Hughes, Amino Acids, Peptides and Proteins in Organic Chemistry: Building Blocks, Catalysis and Coupling Chemistry; ed by. A. B. Hughes: Wiley-VCH, Weinheim, 2011, pp. 83-114.
- Y. Ohashi, R. Onuma, T. Nagakuma, T. Ogawa, R. Naude, K. Nokihara, K. Muramoto, Antioxidant properties tripeptides revealed by a comparison of six different assays, Food Sci. Technol. Res., 2016, 21, 695-704.
- QY. Wei, H. Jiang, JX. Zhang, C. Zhang, PF. Guo, Antimicrobial activities of the cinnamoyl amide of amino acid derivatives, Asian J. Chem., 2012, 24, 2383-2388.
- H. G. Ghalehshahi, S. Balalaie, A. Aliahmadi, R. Moghimi, Synthesis of 4-N-α-coumaryl amino acids and investigation of their antioxidant, antimicrobial activities and fluorescence spectra. Amino Acids., 2018, 50, 1461-1470.
- Y. Shivaraj, Malenahalli, Naveen, R. Giriyapura, Vijayakumar, Doyijode, B. Aruna Kumar, Design, Synthesis and Antibacterial Activity Studies of Novel Quinoline Carboxamide Derivatives, J. K. Chem. Soc., 2013, 57, 241-245.
- A. Pinazo, M.A. Manresa, A.M. Marques, M. Bustelo, M.J. Espuny, L. Pérez, Amino acid–based surfactants: New antimicrobial agents, Adv. Colloid Interfac Sc., 2016, 228, 17–39.
- R. Ammar, M. Zayed, Sulaiman. PH-titration, synthesis and antimicrobial activity of Co(II) complexes of Girard T and amino acids, Life Sci J., 2014, 11, 437-443.
- I. Irwansyah, Li YQ, Shi W, Qi D, Leow WR, Tang MBY, Li S, Chen X, Gram-positive antimicrobial activity of amino acid-based hydrogels, Adv. Mater., 2015, 27, 648-654.
- Junyong Zhang, Xiaokang Ke, Chao Tu, Jun Lin, Jian Ding, Liping Lin, HoongKun Fun, Xiaozeng You, Zijian Guo, Novel Cu(II)-quinoline carboxamide complexes: Structural characterization, cytotoxicity and reactivity towards 5′-GMP, BioMetals., 2003, 16, 485-496.
- Y. Filali Baba, Yusuf Sert, Youssef Kandri Rodi, Sonia Hayani, Joel T. Mague, Damien Prim, Jerome Marrot, Fouad Ouazzani Chahdi, Nada Kheira Sebbar, El Mokhtar Essassi, Synthesis, crystal structure, spectroscopic characterization, Hirshfeld surface analysis, molecular docking studies and DFT calculations, and antioxidant activity of 2-oxo-1,2-dihydroquinoline-4-carboxylate derivatives, J. Mol. Struct., 2019, 1188, 255-268.
- Y. Filali Baba, Y. Kandri Rodi, K. Misbahi, F. Chahdi Ouazzani, A. Kerbal, E.M. Essassi, Synthesis and reactivity of new heterocyclic systems derived from quinoline, J. Mar. Chim. Heterocycl., 2014, 13: 72-80.
- M. Brenner, W. Huber, Preparation of a-amino acid esters by alcoholysis of the methyl esters, Helv. Chim. Acta, 1953, 36, 1109-1115.
- S.Y. Han, Y.A. Kim, Recent development of peptide coupling reagents in organic synthesis, Tetrahedron, 2004, 60, 2447–2467.
- MRS Zaidan, A. Noor Rain, AR. Badrul, A. Adlin, A. Norazah, I. Zakiah, In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method, Trop. Biomed., 2005, 22, 165-170.
- S. Bouhdid, J. Abrini, A. Zhiri, M.J. Espuny, A. Manresa, Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil, J. Appl. Microbiol., 2009, 106, 1558-1568.
DOI: http://dx.doi.org/10.13171/mjc941911231077sc
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
