Improvement of glyphosate adsorption using new composites based on Ghassoul and chitosan: Kinetics and equilibrium study
Abstract
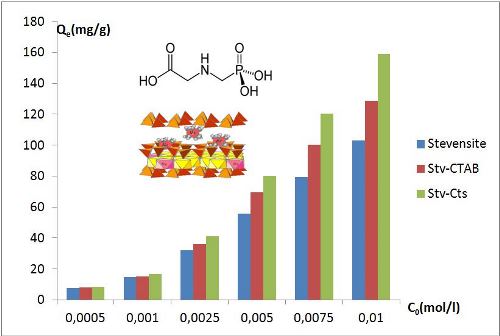
Clay materials combined with other compounds are intensely used in several fields. The present study focuses on the synthesis of organoclays and nanocomposites based on the intercalation of surfactant and biopolymer into swelling Moroccan clay. The produced materials were used in the removal of glyphosate from the aqueous medium. The organoclay was obtained by the intercalation of cetyltrimethylammonium bromide (CTAB) in the interlamellar space of stevensite, and the nanocomposite was fabricated by the direct interaction between the stevensite and chitosan. These materials were characterized by mean X-Ray Diffraction, Fourier Transform Infra-Red Spectroscopy (FTIR), Thermal Analysis and Transmission Electron Microscopy (TEM). The results showed that the stevensite is the dominant clay mineral of Ghassoul and confirmed that the nanocomposite has an intercalated clay structure. The batch mode was accomplished to quantify the glyphosate removal capacity from aqueous solution by these materials. The enhancement of hydrophobic properties has promoted the retention of the herbicide. The pseudo-second-order kinetic model of adsorption was the most suitable to describe the process of adsorption and the Freundlich isotherm equation fitted satisfactory the adsorption isotherm data. The capacity of adsorption was more outstanding for the nanocomposite chitosan/Ghassoul and reached a significant value of 159.10 mg.g-1. The nanocomposites based on chitosan/Ghassoul could be considered as promising materials for treatments of pesticide-contaminated water.
Full Text:
PDFReferences
J. E. Franz, M. K. Mao, J.A. Sikorski, Glyphosate: a unique global herbicide. ACS monograph 189, American Chemical Society, Washington, DC, 1997, p. 653.
J. Malik, G. Barry, G. Kishore, The herbicide glyphosate. Biofactors, 1989, 2, 17-25.
M. Maroni, C. Colosio, A. Ferioli, A. Fait, Biological Monitoring of Pesticide Exposure: a review. Introduction. Toxicology, 2000, 143, 1-118.
G. M. Williams, R. Kroes, I. C. Munro, Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol, 2000, 31 ,117-165
C. Cox, Glyphosate, J. Pestic. Reform, 1991,11,2, 35–38.
K. Solomon, D. Thompson, Ecological risk assessment for aquatic organisms from over-water uses of glyphosate, J. Toxicol. Environ. Health B, 2003, 6, 3, 289–324.
R.A. Relyea, The lethal impact of Roundup on aquatic and terrestrial amphibians, Ecol. Appl., 2005,15, 4, 1118–1124.
GHS/CLP-Regulation (EC) No 1272, Annex VI, Table 3–1, List of harmonised classification and labelling of hazardous substances, 2008, pp. 235.
T. F. Speth, Glyphosate removal from drinking water, J. Environ. Eng., 1993,119, 1139–1157.
Y. Hu, Y. Zhao, B. Sorohan, Removal of glyphosate from aqueous environment by adsorption using water industrial residual, Desalination, 2011, 271, 150–156.
S. S. Salih, A. Mahdi, M. Kadhom, T.K. Ghosh, Competitive adsorption of As (III) and As (V) onto chitosan/diatomaceous earth adsorbent; J. Environ. Chem. Eng., 2019, 7, 5, 103407
S. S. Salih, T. K. Ghosh, Preparation and Characterization of Chitosan-Coated Diatomaceous Earth for Hexavalent Chromium Removal, Environ. Processes, 2018, 5,1, 23-39.
S.S. Salih, T.K. Ghosh, Preparation and characterization of bioadsorbent beads for chromium and zinc ions adsorption. Cogent Environ. Sci. 201, 3, 1, 1401577.
A.L. Gimsing, O. K. Borggaard, Phosphate and glyphosate adsorption by hematite and ferrihydrite and comparison with other variable-charge minerals, Clays Clay Miner., 2007, 55, 108–114.
A. Gautam, A. Kshirsagar, R. Biswas, S. Banerjee, P. K. Khanna, Photodegradation of organic dyes based on anatase and rutile TiO2 nanoparticles, RSC Adv., 2016, 6, 2746-2759
S. Azarkan, A. Peña, K. Draoui, C. Ignacio Sainz-Diaz, Adsorption of two fungicides on natural clays of Morocco, App. Clay Sci., 2016, 123, 37-46.
M. Ahrouch, J. M. Gatica, K. Draoui, D. Bellido, H. Vidal, Lead removal from aqueous solution by means of integral natural clays honeycomb monoliths, J. Hazard. Mater., 2019, 365, 519-530.
Y. J. Tang, J .Y. Jia, X .D. Xie, Environment significance of clay minerals. Earth Science Frontiers, 2002, 9, 2, 337–344.
Y. Bentahar, C. Hurel, K. Draoui, S. Khairoun, N. Marmier, Adsorptive properties of Moroccan clays for the removal of arsenic(V) from aqueous solution, App. Clay Sci., 2016,119, 385–392
L. Bouna, B. Rhouta, F. Maury, A. Jada, F. Senocq and M.C. Lafont Photocatalytic activity of TiO2/stevensite nanocomposites for the removal of Orange G from aqueous solutions, Clay Miner., 2014, 49, 365–376.
R. Ozola, A. Krauklis, J. Burlakovs, M. Klavins, Z. Vincevica-Gaile W. Hogland, Surfactant-modified clay sorbents for the removal of p-nitrophenol, Clays and Clay Miner. 2019, 67, 2, 132–142.
H. Azejjel, C. del Hoyo, K. Draoui, M.S. Rodrıguez-Cruz, M.J. Sanchez-Martın, Natural and modified clays from Morocco as sorbents of ionizable herbicides in an aqueous medium, Desalination, 2009, 249, 1151-1158.
A. Nicoleta, N. Suciu, E. Capri, Adsorption of chlorpyrifos, penconazole and metalaxyl from aqueous solution by modified clays, J. Environ. Sci. Health Part B, 2009, 44, 525–532,
M. Rinaudo, Chitin and Chitosan Properties and Applications, Prog. Polym. Sci. 2006, 31, 603–632.
M. N. Avi Kumar, A review of chitin and chitosan applications React. Funct. Polym., 2000,46, 1–27.
S. B. Kim, Y. J. Kim, T. L. Yoon, S. A. Park, I. H. Cho, E. J. Kim, I. A. Kim, J. W. Shin, Biomaterials, The characteristics of hydroxyapatite-chitosan-PMMA bone cement, 2004, 25, 5715–5723.
L. Z. Zhao, C. H. Zhou, J. Wang, D. S. Tong, W. H. Yu, H. Wang, Recent advances in clay mineral-containing nanocomposite hydrogels, Soft Matter, 2015,11, 9229-9246
S.F. Wang, L. Shen, W.D. Zhang, Y.J. Tong, Preparation and mechanical properties of chitosan/carbon nanotubes composites, Biomacromolecules, 2005, 6, 3067–3072.
J. Zhang, E. Manias, C.A. Wilkie. Polymerically modified layered silicates: an effective route to nanocomposites, J. Nanosci. Nanotechnol., 2008, 8,1597–615.
M. Jaber, J. Miéhé-Brendlé, Formation of organoclays by a one-step synthesis, Solid State Sci. 2005, 7, 5,610-615
C. Hu, P. Zhu, M. Caia, H. Hub, Q. Fub Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: Kinetic, thermodynamic and equilibrium studies, App. Clay Sci., 2017, 143, 320–326.
F.A.R. Pereira, K. S. Sousa, G.R.S. Cavalcanti, D.B. França, L.N.F. Queiroga, I.M.G. Santos, M.G. Fonseca, M. Jaber, Green biosorbents based on chitosan-montmorillonite beads for anionic dye removal Journal of Environ. Chem. Engin., 2017, 5, 3309–3318
H. Azejjel, J. M. Ordax, K. Draoui, M. S. Rodríguez-Cruz, M. J. Sánchez-Martín, Effect of cosolvents on the adsorption of ethofumesate by modified Moroccan clays, App. Clay Sci., 2010, 49, 120-126
B. Rhouta, H. Kaddami., J. Elbarqy, M. Amjoud, L. Daoudi., F. Maury, F. Senocq, A. Maazouz, J. F Gerard, Elucidating the crystal-chemistry of Jbel Rhassoul stevensite (Morocco) by advanced analytical techniques, Clay minerals, 2008, 43, 393-404
M. Darder, Mon Colilla, Eduardo Ruiz-Hitzky, Biopolymer−Clay Nanocomposites Based on Chitosan Intercalated in Montmorillonite, Chem. Mater.,2003, 15 ,20 , 3774-3780
Y. S. Han, S. H. Lee, K. H. Choi, Preparation and characterization of chitosan–clay nanocomposites with antimicrobial activity, J. of Phys. Chem. Solids, 2010,71,464–467
J. Madejova, FTIR techniques in clay mineral studies, Vib. Spectrosc., 2003, 31, 1-10
C. Branca, G. D'Angelo, C. Crupi, K. Khouzami, S. Rifici, G. Ruello, U. Wanderlingh, Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR ATR study on chitosan and chitosan/clay films, Polymer, 2016, 99, 614-622
S. Lagergren., B. K. Svenska., Vetenskapsakad. Handl., 1898, 24,1-39.
M. Ӧzacar, Ỉ. A. Şengil, Adsorption of reactive dyes on calcined alunite from aqueous solutions, J. Hazard. Mater. 2003, 98, 211-224.
Y. S. Ho, G. McKay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34, 451-465.
Y.S. Ho, G. McKay, A multi-stage batch sorption design with experimental data, Adsorpt. Sci. Technol. 1999,17, 233-243
M. J. D. Low, Kinetics of chemisorption of gases on solids, Chem. Rev., 1960, 60, 267-312.
W.J. Weber, J.C. Morris, Advances in water pollution research: removal of a biologically resistant pollutant from wastewater by adsorption. International Conference on Water Pollution Symposium. Pergamon, Oxford 1962, 2. 231-266
C. H. Giles, T. H. MacEwan, S. N. Nakhwa, D. Smith, Studies in adsorption: Part IX. A system of classification of solution adsorption isotherms and its use on the diagnosis of adsorption mechanisms and in the measurement of specific areas of soils. J. Chem. Soc., 1960, 111, 3973-3993.
G. A. Khoury, T. C. Gehris, L. Tribe, R. M. Torres Sánchez, M. S. Afonso, Glyphosate adsorption on montmorillonite: An experimental and theoretical study of surface complexes, App. Clay Sci., 2010, 50,167–175
L. Bounab, K. Draoui, M. Ahrouch, M. Hadri, D. Bouchta, A. Barhoun, An effective functionalized Moroccan bentonite: Application for green remediation of m-Cresol, J. Mater. Environ. Sci., 2017, 8, 1, 244-256
S.S. Mayakaduwa, P. Kumarathilaka, I. Herath, M. Ahmad, M. Al-Wabel, Y.S. Ok, A. Usman, A. Abduljabbar, M. Vithanage. Equilibrium and kinetic mechanisms of a woody biochar on aqueous glyphosate removal Chemosphere, 2016, 144, 2516–2521
A. Khenifi, Z. Derriche, C. Mousty, V. Prévot, C. Forano, Adsorption of Glyphosate and Glufosinate by Ni2AlNO3 layered double hydroxide Applied Clay Science 2010, 47, 362–371
I. Langmuir, The adsorption of gases on plane surfaces of glass mica and platinum, J. Am. Chem. Soc. 1918, 40, 1361–1403.
H. Freundlich, Ueber die Adsorption in Loesungen. Engelmann, Leipzig. 1906.
M. J. Temkin, V. Pyzhev, Recent Modifications to Langmuir Isotherms, Acta Physiochim, URSS, 1940, 12, 217-222.
DOI: http://dx.doi.org/10.13171/mjc9419111121087kd
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
