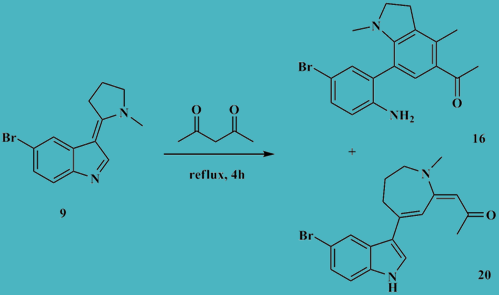
Study of the reaction of (Z)-5-bromo-3-(1-methylpyrrolidin-2- ylidene)-3H-indole with pentane-2,4-dione
Abstract
5-yl)ethanone. Each product is believed to be derived from initial protonation of (Z)-5-bromo-3-(1-methylpyrrolidin-2-ylidene)-3H-indole by the diketone followed with nucleophilic diketone-C-3- addition at the C-2 of the 3H-indolium cation.
Full Text:
PDFReferences
G. A. Youngdale, D. G. Anger, W. C. Anthony, J. P. Da Vanzo, M. E. Greig, R. V. Heinzelman, H. H. Keasling, J. Szmuszkovicz, J. Med. Chem. 1964, 7, 415–427.
M. Harris, J. A. Joule, J. Chem. Res. (S), 1978, 25, (M), 1978, 470-483.
D. I. Bishop, I. K. Al-Khawaja, J. A. Joule, J. Chem. Res. (S), 1981, 361, (M), 1981, 4279–4290.
D. I. Bishop, I. K. Al-Khawaja, F. Heatley, J. A. Joule, J. Chem. Res. (S), 1982, 152, (M), 1982, 1766–1776.
I. K. Al-Khawaja, R. L. Beddoes, D. I. Bishop, R. J. Cernik, J. A. Joule, O. S. Mills, J. Chem. Res. (S), 1984, 296-297, (M), 1984, 2738–2767.
M. Helliwell, M. Aghazadeh, M. M. Baradarani, J. A. Joule, Acta Cryst, 2009, E65, 3114.
M. Salas, I. K. Al-Khawaja, M. J. Thomas, J. A. Joule, J. Chem. Res. (S), 1988, 218, (M), 1988, 1666-1675.
M. Helliwell, M. Aghazadeh, M. M. Baradarani, J. A. Joule, Acta Cryst, 2010, E66, o1532.
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
