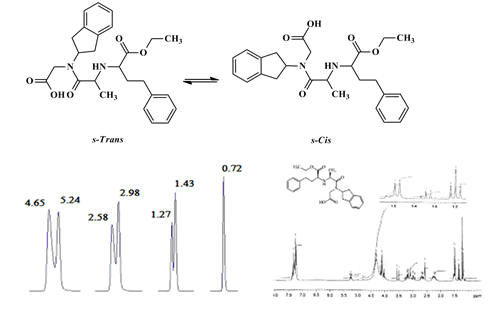
The study of separation of delapril’s s-cis/s-trans isomers and development and validation of an analytical method for the estimation of delapril by reversed-phase liquid chromatography
Abstract
Full Text:
PDFReferences
- E. Redenti, M. Zanol, G. Amari, P. Ventura, G. Fronza, A. Bacchi and G. Pelizzi, Il Farmaco, 1998, 53, 214-223.
- R. Razzetti and D. Acerbi, American Journal of Cardiology, 1995, 75, 7F-12F.
- V. Todeschini, M. S. Sangoi and N. M. Volpato, European Journal of Mass Spectrometry, 2011, 17, 3, 287-296.
- V. Todeschini, A. S. Meira, M. S. Sangoi, A. B. Barth, C. S. Paim and N. M. Volpato, Journal of Liquid Chromatography and Related Technologies, 2012, 35, 5, 603-620.
– V. Todeschini, A. T. Barden, L. L. Sfair, M. S. Sangoi and N. M. Volpato, ActaChimicaSlovenica, 2013, 60, 2, 335–342.
– V. Todeschini and M. S. Sangoi,Journal of AOAC International, 2014, 97, 1, 114-120.
– V. Todeschini, P. R. de Oliveira, L. S. Bernardi, R. L. Pereira, C. E. M. de Campos, M. A. S. Silva and N. M. Volpato, Journal of Thermal Analysis and Calorimetry, 2014, 115, 2295–2301.
- P. J. Rudzki, K. Buś, H. Ksycińska,and K. Kobylińska, Journal of Pharmaceutical and Biomedical Analysis, 2007, 44, 356–367.
- O. Trapp, G. Schoetz,and V. Schurig, Chirality, 2001, 13, 403-417.
-A. Ben Amar, M. Abidi,and N. Ben Hamida, Journal de la Société Chimique de Tunisie, 2013, 15,9-17.
-D. Perrett and P. L. Drury, Journal of Liquid Chromatography, 1982, 5, 97-110.
- T. Kato, AnalyticaChimicaActa, 1985, 175, 339-344.
- S. Gustafsson, B.M. Eriksson, and I. Nilsson, Journal of Chromatography, 1990, 506, 75–83.
- J. Salamoun and K. Slais, Journal of Chromatography, 1991, 537, 249–264.
- R. Bhushan and D. Gupta, Journal of Chromatography B, 2006, 837, 133–137.
- T. Nishikawa, Y. Hayashi, S. Suzuki, H. Kubo, and H. Ohtani, Analytical Sciences, 1996, 12, 561-564.
-A.S. Rathore, and C. Horváth, Journal of Chromatography A, 1997, 787, 1-12.
- R. Ledger and E. Stellwagen, Journal of Pharmacy and Pharmacology, 2005, 57, 845–850.
- R. Cirilli, C. Di Bugno, and F. La Torre, Chromatographia, 1999, 49, 628-634.
- H. Trabelsi, S. Bouabdallah, S. Sabbah, F. Raoufi, and K. Bouzouita, Journal of Chromatography, 2000, 871, 189-199.
- S. Bouabdallah, H. Trabelsi, K. Bouzouita, and S. Sabbah, Journal of Biochemical and Biophysical Methods, 2002, 54, 391–405.
- S. Bouabdallah, H. Trabelsi, T. Ben Dhia, S. Sabbah, K. Bouzouita,and R. Khaddar, Journal of Pharmaceutical and Biomedical Analysis, 2003, 31, 731-741.
- S. Bouabdallah, H. Trabelsi, M. T. Ben Dhia, and N. Ben Hamida, Chromatographia, 2012, 75, 1247-1255.
- S. Bouabdallah, M. T. Ben Dhia,and M. R. Driss, International Journal of Analytical Chemistry, 2014, 1-8.
- S. Gebauer, S. Friebe, G. Scherer, G. Gübitz, and G-J. Krauss, Journal of Chromatographic Science, 1998, 36, 388-394.
- S. Gikas, F. Tsopelas, C. Giaginis, J. Dimitrakopoulos, T. Livadara, H. Archontaki, and A. Tsantili-Kakoulidou, Journal of Pharmaceutical and Biomedical Analysis, 2008, 48, 739–743.
- N. Tanaka, H. Goodell, and B.L. Karger, Journal of Chromatography, 1978, 158, 233–248.
- X-Z. Qin, J. DeMarco,and D. P. Ip, Journal of Chromatography, 1995, 707, 245–254.
- A. Kálmán, F. Thunecke, R. Schmidt, P.W. Schiller, and C. Horváth, 1996, 729, 155–171.
-medicine.cug.net/drug/05/05_03_02.htm.
- United States Pharmacopeia, 29th Edition. United States Pharmacopeial Convention, Inc, Twinbrook Parkway, Rockville, MD, USA.pp. 1623, 2675-2693, 3050-3053, 3392-3394, 2006.
- US Food and Drug Administration, Center for Drug Evaluation and Research, Guidance for Industry: Analytical Procedures and Methods Validation. Chemistry, Manufacturing, and Controls Documentation DRAFT GUIDANCE, Rockville, MD, 2000.
http://www.fda.gov/CDER/GUIDANCE/2396dft.pdf.
- International Conference on Harmonization (ICH) of Technical Requirements for the registration of Pharmaceuticals for Human Use, Guideline for Industry Q2A. Text on Validation of Analytical Procedures, Geneva: 1995.
http://www.fda.gov/cder/Guidance/ichq2a.pdf.
DOI: http://dx.doi.org/10.13171/mjc.3.4.2014.15.08.16
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
