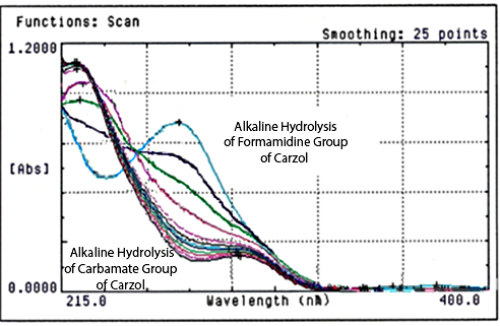
Kinetic study by UV Spectrophotometry of Carzol degradation in aqueous medium
Abstract
Full Text:
PDFReferences
-C. C. Aguilar, H. Villanueva and Childers,Florida Entomological Society,2001, 84, 391-401.
-T. G.Grout, G. I.Richards, P. R.Stephen,Exp. Appl. Acarol.,1997, 21, 171-177.
-I.Khan, J. G.Morse, J. Agric. Entomol.,1997, 14,409-420.
-D. L. Hardman, J. M.Franklin, J. L. Moreau, Pest Manag. Sci., 2003, 59, 1321-1332.
-R. W. Hu, V. Petay, J. Fournier, J. Agric. Food Chem.,1996, 44,181-184.
-J.Wang, W.Cheung, D.Grant,J. Agric. Food Chem.,2005, 53, 528-537.
-S. Suwansa-Ard,P. Kanatharana, P. Asawatreratanakul,C. Limsakul, B. Wongkittisuksa, P. Thavarungkul, Biosens. Bioelectron.,2005, 21, 445-454.
-G. Lin,H. G. Chen,C. S. Yeh,P. C. Lu, J. Biochem. Mol. Toxicol., 2005, 19, 234-243.
-J.D. Marty, C. Fournier, M. Mauzac, I. Rico-Lattes, A. Lattes. Liquid Crystals., 2002, 29, 529.
-L. Hiripi, L. Nagy, M. R. Hollingworth,Acta Biol. Hung.,1999, 50, 81-87.
-V. Visentin,N. Morin,E. Fontana,D. Prevot,J. Boucher, I. Castan, P. Valet, D. Grujic, C. Carpene, J. Pharmacol. Exp. Ther., 2001, 299, 96-104.
-M. Sogorb, E. Vilanova,ToxicologyLetters., 2002, 128, 215.
-F. Testud, R. Garnier, B. Delemotte, Toxicologie humaine des produits phytosanitaires,Paris, 2001, 67-90.
-K. T. Douglas, A. Williams,Chem. Rev., 1975, 75, 627.
-C. B. Divito, S. Davias, S. Masoudi, C. N. Muhoro, J. Agric. Food. Chem., 2007, 55, 5377.
-A. Cegan, J. Slosar, M. Vecera, Collect. Czech. Chem. Commun., 1980, 45, 1065-1071.
-M. Bergon, J. P. Calmon, Bull. Soc. Chim., 1976, 797.
-M. L. Bender, R. B. Hober. Org. Chem., 1965, 30, 3975.
-N. Ben Hamida, M. Smaali, S. Sabbah, J. Soc. Chim. Tun., 2005, 7, 143-157.
-F. Boujelbane, N. Ben Hamida, J. Soc. Chim. Tun., 2007, 9, 121-132.
-F. Boujelbane, N. Ben Hamida, J. Soc. Chim. Tun., 2008, 10, 39-51.
-R.Ouertani, L.Latrous El Atrache, N.BenHamida, International Journal of Chemical Kinetics.,2012, 45, 118-124.
-R. Ouertani, L. Latrous El Atrache, N. Ben Hamida, Progress in Reaction Kinetics and Mechanism.,2012, 37, 183-192.
-M. Bergon, N. Ben Hamida, P. J. Calmon, J. Agric. Food. Chem., 1985, 33, 577-583.
-I. Christenson, Acta. Chem. Scan.,1964, 18, 904.
-A. F. Williams, L. N. Frost, J. Chem. Soc., Perkin II, 1973, 1719.
-R. Ouertani, A. Gadhgadhi, N. Ben Hamida, J. Soc. Chim. Tun., 2013, 15, 29-38.
-N. Ben Hamida, M. Smaali, S. Sabbah., J. Soc. Chim. Tun., 2004, 6, 45.
DOI: http://dx.doi.org/10.13171/mjc.3.4.2014.20.07.14
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
