Removal of Evans Blue and Yellow thiazole dyes from aqueous solution by Mg-Al-CO3 Layered Double Hydroxides as anion-exchanger
Abstract
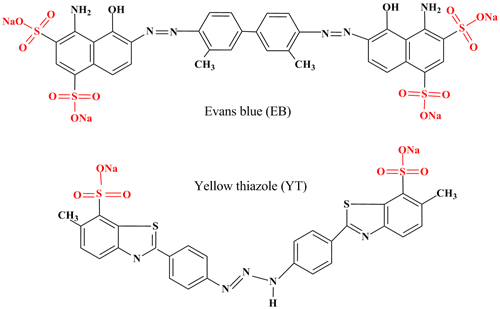
Mg-Al-CO3 Layered double hydroxide (LDH) was prepared by co-precipitation method at constant pH, and subsequently used to remove Evans Blue (EB) and Yellow thiazole (YT) dyes from aqueous solutions.
The obtained material was characterized by powder X-ray diffraction (PXRD), Fourier transform infrared spectroscopy (FTIR), thermal analysis and BET. The kinetic and equilibrium aspects of sorption of the anionic dyes from aqueous solution by Mg-Al-CO3 were investigated in batch mode. The sorption kinetic data were found to be consistent with the pseudo-second-order model. Data for YT and EB sorption by Mg-Al-CO3 were fitted better by the Langmuir equation than by the Freundlich equation based on the determination coefficient values R2. The maximum adsorption capacities of 222.2 mgg-1 for Yellow thiazole and 107.5 mgg-1 for Evans blue. The thermodynamic parameters including Gibbs free energy (ï„ G°), standard enthalpy change (ï„ H°), and standard entropy change (ï„ S°) for the process were calculated. The negative ï„ G° value indicates the spontaneity of the removal process.
Full Text:
PDFReferences
- M. X. Zhu, Y. P. Li, M. Xie, H. Z. Xin, .J. Hazard. Mat. 2005, B120, 163–171.
- O. Gulnaz, A. Kaya, F. Matyar, B. Arikan, J. Hazard. Mat. 2004, B108, 183–188.
- W. T. Tsai, C. Y. Chang, M. C. Lin, S. F. Chien, H. F. Sun, M.F. Hsieh, Chemosphere 2001, 45, 51-58.
- M. Özacar, I. A. Sengil, Colloids and Surfaces A: Physicochem. Eng. Aspects 2004, 242, 105-113.
- N. Thinakaran , P. Baskaralingam , M. Pulikesi , P. Panneerselvamc, S. Sivanesan, J. Hazard. Mat.2008, 151, 316-322.
- P. C. C. Faria, J.J. Mórfão, M. F. R. Pereira, Water Res. 2004, 38, 2043-2052.
- A. Mittal, J. Mittal, A. Malviya, D. Kaur, V. K. Gupta,J. Colloid Interface Sci.2010, 343, 463-473.
- V. Rochera, A. Beea, J. M. Siauguea, V. Cabuila, J. Hazard. Mat.2010, 178, 434-439.
- S. J. Allen, Q. Gan, R. Matthews, P A. Johnson, J. Colloid Interface Sci. 2005, 286, 101-109.
- Z. Rawajfih, N. Nsour, J. Colloid Interface Sci. 2006, 298, 39-49.
- M. Mana, M. S. Ouali, L. C. de Menorval, J. Colloid Interface Sci.2007,307, 9-16.
- A. H. Chen, Y. Y Huang, J. Hazard. Mat. 2010, 177, 668-675.
- A. R. Auxilio, P. C. Andrews, P. C. Junk, L. Spiccia, Dyes and Pigments 2009, 81, 103-112.
- M. Bouraada, M. Lafjah, M. S. Ouali, L. C. de Ménorval, J. Hazard. Mat. 2008, 153, 911-118.
- M. Bouraada, F. Belhalfaoui, M. S. Ouali, L.C. de Ménorval, J. Hazard. Mat. 2009, 163, 463-467.
- F. Cavani, F. Trifiro, A. Vaccari, Catal. Today1991, 11, 173-301.
- F. Kooli, W. Jones, Inorg. Chem. 1995, 34, 6237-6238.
- J. Olanrewaju, B. L. Newalkar, C. Mancino, S. Komarneni, Mat. Lett.2000, 45,307-310.
- W. T. Reichle, Solid State Ionics 1986, 22, 135-141.
- T. Toraishi, S. Nagasaki, S. Tanaka, Appl. Clay Sci. 2002, 22, 17-23.
- Y. Zhixin, D. Chen, M. R Torbjørn, V. Esther, O. Fernández, A. Holmen, Appl. Catal. A. General., 2008, 338, 136-146.
- V. Mas, M. L. Dieuzeide, M. Jobbágy, G. Baronetti, N. Amadeo, M. Laborde, Catal. Today 2008, 133, 319-323.
- D. M. Meira, G. G. Cortez1, W. R. Monteiro, J. A. J. Rodrigues, Brazilian J. Chem. Eng. 2006, 23, (03), 351-358.
- H. Fu-An, L. M. Zhang, J. Colloid Interface Sci. 2007, 315, 439-444.
- G. P. Gillman, M. A. Noble, M. D. Raven, Appl. Clay Sci. 2008, 38, 179-186.
- L. Forni, G. Fornasari, F. Trifiro, Microp. Mesop. Mat. 2008, 107, 39-45.
- L. Liang, P. Sun, G. Zhengyu, Du. Hangeng, X. Pang, X. Tao, R. Xu, L. Xu, J. Hazard. Mat. 2009, 161, 1444-1449.
- J. Das, B. S. Patra, N. Baliarsingh, K.M. Parida, J. Colloid Interface Sci. 2007, 316, 216-223.
- M. Del Arco, E. Cebadera, S. Gutierrez, C. Martin, M. J. Montero, V. Rives, J. Rocha, M. M. Sevilla, J. Pharm Sci. 2004, 93, 1649-1658.
- H. Zhao, K. L. Nagy, J. Colloid Interface Sci. 2004, 274, 613-624.
- C. A. S. Barbosa, P. M. Dias, A. M. C. Ferreira, V. R. L. Constantino, Appl. Clay Sci. 2005, 28, 147-158.
- R. Dula, K. Wcislo, J. Stoch, B. Grzybowska, E. M. Serwicka, F. Kooli, K. Bahranowski, Appl. Catal. A: Gen. 2002, 230-281.
- J. T. Kloprogge, D. Wharton, L. Hickey, R.L. Frost, Am. Mineral 2002, 87, 623-629.
- S. Lagergren, Handl. 1898, 24, 1-39.
- C. Namasivayam, K. Kadirvelu, Carbon1999, 37, 79-84.
- C.W. Cheung, J.F. Porter, G. McKay, Sep. Purif. Technol.2000, 19, 55–64.
- N. Chiron, R. Guilet, E. Deydier, Water Res. 2003, 37, 3079-3086.
- R. C. Francis, A. Leuteritz, U. Wagenknecht, D. Ehnichen, L. Häußler, G. Heinrich, Appl. Clay Sci. 2008, 38, 153-164.
- Y.S. Ho, G. Mckay, Chem. Eng. J. 1998, 70, 115-124.
- C. Namasivayam, S. Sumithra, Ind. Eng. Chem. Res. 2004, 43, 7581-7587.
- Y. Bulut, H. Aydin, Desalination2006,194, 259-267.
- G. Bascialla, A. E. Regazzoni, Colloids and Surfaces A: Physicochem. Eng. Aspect 2008, 328, 34-39.
- E. Géraud, M. Bouhent, Z. Derriche, F. Leroux, V. Prévot, C. Forano, J. Phys. Chem. Solids 2007, 68, 818-823.
- N. K. Lazaridis, T.D. Karapantsios, D. Georgantas, Water. Res., 2003, 37, 3023-3033.
- L. Lian, L. Guo, C. Guo, J. Hazard. Mat. 2009, 161, 126-131.
- N. Benselka, A. Hadj, A. Bentouami, Z. Derriche, N. Bettahar, L.-C de Ménorval, Chem. Eng. J.2011, 169, 231-238.
- F. Renault, N. Morin-Crini, F. Gimbert, P. Badot, G. Crini, Bioresource Tech. 2008, 99, 7573-7586.
- H. Qiuhong, X. Zhiping, Q. Shizhang, F. Haghseresht, M. Wilson, G.Q. Lu, J. Colloid Interface Sci. 2007, 308, 191-199.
DOI: http://dx.doi.org/10.13171/mjc.3.3.2014.01.06.18
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
