Experimental and theoretical study on corrosion inhibition of new synthesized menthone derivatives (Menthopyrazole compounds) for mild steel in 1 M HCl solution
Abstract
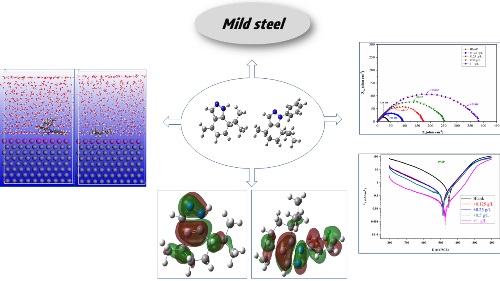
The aim of the present work is to evaluate the protective ability of newly menthone derivatives 7-isopropyl-4-methyl-4,5,6,7-tetrahydro-1H-indazole (HMP) and 7-isopropyl-4-methyl-1-phenyl-4,5,6,7-tetrahydro-1H-indazole (PMP) as mild steel corrosion inhibitors in 1M HCl, which may get applications as eco-friendly corrosion inhibitors in acidizing processes in the industry. The corrosion attitude of mild steel at various concentrations of the inhibitors was examined in the range from 298 to 328 K. An increase in the inhibitory effectiveness in acidic solution at higher concentration and temperature follow the existence of inhibitors. The aspect of adsorption mixed type of inhibitors was highlighted by the Polarization curves. The adsorption of HMP and PMP is in line with the Langmuir isotherm model. Theoretical indices study of both inhibitors (HMP and PMP) via CDFT (the conceptual density functional theory) has been studied. To search for the best spatial configuration of steel/inhibitor a Monte Carlo simulation studies were applied.
Full Text:
PDFReferences
- M.A. Bedair, S.A. Soliman, M.A. Hegazy, I.B. Obot, A.S. Ahmed, Empirical and theoretical investigations on the corrosion inhibition characteristics of mild steel by three new Schiff base derivatives, Journal of Adhesion Science and Technology, 2019, 33,1139-1168.
- A.K. Singh, S. Thakur, B. Pani, B. Chugh, H. Lgaz, I.-M. Chung, P. Chaubey, A.K. Pandey, J. Singh, Solvent-free microwave assisted synthesis and corrosion inhibition study of a series of hydrazones derived from thiophene derivatives: Experimental, surface and theoretical study, Journal of Molecular Liquids, 2019, 283, 788–803.
- J. Zhang, 2-(3H-Imidazol-4-yl)-ethylamine as a Green Corrosion Inhibitor for Q235 Steel in Hydrochloric Acid, Int. J. Electrochem. Sci., 2020, 15, 1437–1449.
- A. Singh, K.R. Ansari, M.A. Quraishi, S. Kaya, Theoretically and experimentally exploring the corrosion inhibition of N80 steel by pyrazol derivatives in simulated acidizing environment, Journal of Molecular Structure, 2020, 127685.
- R.A. Rikkouh, T. Douadi, H. Hamani, M. Al-Noaimi, S. Chafaa, Inhibition effect of 4, 4′-thio bis ${$N-[(E)-phenol-3-ylmethylidene] aniline$}$ on the corrosion of mild steel in HCl solution under stagnant condition and hydrodynamic flow, Journal of Adhesion Science and Technology, 2020, 1–26.
- Z. Rouifi, M. Rbaa, A.S. Abousalem, F. Benhiba, T. Laabaissi, H. Oudda, B. Lakhrissi, A. Guenbour, I. Warad, A. Zarrouk, Synthesis, Characterization and Corrosion Inhibition Potential of Newly Benzimidazole Derivatives: Combining Theoretical and Experimental Study, Surfaces and Interfaces. 2020, 100442.
- J. Zhang, 2-(3H-Imidazol-4-yl)-ethylamine as a Green Corrosion Inhibitor for Q235 Steel in Hydrochloric Acid, Int. J. Electrochem. Sci., 2020, 15, 1437–1449.
- E.K. Ardakani, E. Kowsari, A. Ehsani, Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 586, 124195.
- A. Sedik, D. Lerari, A. Salci, S. Athmani, K. Bachari, İ. Gecibesler, R. Solmaz, Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Electrochemical and surface morphological studies, Journal of the Taiwan Institute of Chemical Engineers, 2020. https://doi.org/10.1016/j.jtice.2019.12.006.
- Z. Faska, A. Bellioua, M. Bouklah, L. Majidi, R. Fihi, A. Bouyanzer, B. Hammouti, Effect of pulegone and pulegone oxide on the corrosion of steel in 1 M HCl, Monatshefte Für Chemie-Chemical Monthly, 2008, 139, 1417–1422.
- L. Majidi, Z. Faska, M. Znini, S. Kharchouf, A. Bouyanzer, B. Hammouti, Synthesis and anticorrosive effects of epoxy-allylpulegols on steel in molar hydrochloric acid, J. Mater. Environ. Sci., 2010, 1, 219–226.
- S. Kharchouf, L. Majidi, M. Znini, J. Costa, B. Hammouti, J. Paolini, Stereoselective synthesis and corrosion inhibition behaviour of Allyldihydrocarveols on steel in molar hydrochloric acid, Int. J.
Electrochem. Sci., 2012, 7, 10325–10337.
- S. Kharchouf, L. Majidi, M. Bouklah, B. Hammouti, A. Bouyanzer, A. Aouniti, Effect of three 2-allyl-p-mentha-6, 8-dien-2-ols on inhibition of mild steel corrosion in 1 M HCl, Arabian Journal of Chemistry, 2014, 7, 680–686.
- A. Ansari, M. Znini, I. Hamdani, L. Majidi, A. Bouyanzer, B. Hammouti, Experimental and theoretical investigations anti-corrosive properties of Menthone on mild steel corrosion in hydrochloric acid, J. Mater. Environ. Sci., 2014, 5, 81–94.
- Á. Nagy, Density functional. Theory and application to atoms and molecules, Physics Reports, 1998, 298, 1-79.
- C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Physical Review B, 1988, 37, 785.
- W.J. Hehre, L. Radom, P. Schleyer, v. R. Pople, JA Ab initio molecular orbital theory, Wiley: New York, 1986.
- A. Ansari, A. Oubair, M. Znini, Synthesis and Characterization of new Menthopyrazole Compounds derived from Menthone, International Journal of Innovation and Applied Studies, 2018, 24, 1819–1822.
- R.A. Rikkouh, T. Douadi, H. Hamani, M. Al-Noaimi, S. Chafaa, Inhibition effect of 4,4′-thio bis N-[(E)-phenol-3-ylmethylidene] aniline on the corrosion of mild steel in HCl solution under stagnant condition and hydrodynamic flow, Journal of Adhesion Science and Technology, 2020, 1–26.
- P. Hohenberg, W. Kohn, Density Functional Theory (DFT), Phys. Rev., 1964, 136, B864.
- I.B. Obot, D.D. Macdonald, Z.M. Gasem, Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: an overview, Corrosion Science, 2015, 99, 1–30.
- M. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, Ga. Petersson, Gaussian 09, revision a. 02, gaussian, Inc., Wallingford, CT., 2009, 200, 28.
- R.G. Parr, W. Yang, Density Functional Theory of Atoms and Molecules, New York: Oxford Univ, Press, 1989.
- L.R. Domingo, P. Pérez, J.A. Sáez, Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions, RSC Advances, 2013, 3,1486-1494.
- C. Verma, H. Lgaz, D.K. Verma, E.E. Ebenso, I. Bahadur, M.A. Quraishi, Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: a review, Journal of Molecular Liquids, 2018, 260, 99–120.
- M.A. Bedair, The effect of structure parameters on the corrosion inhibition effect of some heterocyclic nitrogen organic compounds, Journal of Molecular Liquids, 2016, 219, 128–141.
- Accelrys Inc., Materials Studio Revision 8.0. San Diego USA (2016), 2016.
- H. Sun, COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds, The Journal of Physical Chemistry B, 1998, 102, 7338-7364.
- L. Afia, O. Hamed, M. Larouj, H. Lgaz, S. Jodeh, R. Salghi, Novel natural-based diazepines as effective corrosion inhibitors for carbon steel in HCl solution: experimental, theoretical and Monte Carlo simulations, Transactions of the Indian Institute of Metals, 2017, 70, 2319–2333.
- A.E.-A.S. Fouda, M.A. Ismail, A.A. Al-Khamri, A.S. Abousalem, Experimental, quantum chemical and molecular dynamic studies on the action of arylthiophene derivatives as acid corrosion inhibitors, Journal of Molecular Liquids, 2019, 111178.
- G. Mouhsine, K. Tarfaoui, M. Ouakki, M. Nehiri, M.E. Touhami, N. Barhada, M. Ouhssine, Valorization of the essential oil of Zingiber officinale by its Use as inhibitor against the corrosion of the carbon steel in acid medium hydrochloric acid 1M, Mediterranean Journal of Chemistry, 2019, 8, 17–24.
- A.K. Satapathy, G. Gunasekaran, S.C. Sahoo, K. Amit, P.V. Rodrigues, Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution, Corrosion Science, 2009, 51, 2848–2856.
- A.A. Ganash, Comparative Evaluation of Anticorrosive Properties of Mahaleb Seed Extract on Carbon Steel in Two Acidic Solutions, Materials, 2019, 12, 3013.
- S. Shahabi, S. Hamidi, J.B. Ghasemi, P. Norouzi, A. Shakeri, Synthesis, experimental, quantum chemical and molecular dynamics study of carbon steel corrosion inhibition effect of two Schiff bases in HCl solution, Journal of Molecular Liquids, 2019, 285, 626-639.
- F. Mansfeld, M.W. Kendig, S. Tsai, Evaluation of corrosion behavior of coated metals with AC impedance measurements, Corrosion, 1982, 38, 478–485.
- M. Benabdellah, A. Aouniti, A. Dafali, B. Hammouti, M. Benkaddour, A. Yahyi, A. Ettouhami, Investigation of the inhibitive effect of triphenyltin 2-thiophene carboxylate on corrosion of steel in 2 M H3PO4 solutions, Applied Surface Science, 2006, 252, 8341–8347.
- G.-N. Mu, X.-H. Li, Q. Qu, J. Zhou, Synergistic effect on corrosion inhibition by cerium (IV) ion and sodium molybdate for cold rolled steel in hydrochloric acid solution, Acta Chim. Sin., 2004, 62, 2386–2390.
- A.M. Badiea, K.N. Mohana, Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2-hydrazino-4, 7-dimethylbenzothiazole in industrial water medium, Corrosion Science, 2009, 51, 2231–2241.
- S. Sankarapapavinasam, M.F. Ahmed, Benzenethiols as inhibitors for the corrosion of copper, Journal of Applied Electrochemistry, 1992, 22, 390-395.
- L. Tang, G. Mu, G. Liu, The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid, Corrosion Science, 2003, 45, 2251–2262.
- M. Murmu, S.K. Saha, N.C. Murmu, P. Banerjee, Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L- 1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study, Corrosion Science, 2019, 146, 134–151.
- A.K. Singh, A.K. Pandey, P. Banerjee, S.K. Saha, B. Chugh, S. Thakur, B. Pani, P. Chaubey, G. Singh, Eco-friendly disposal of expired anti-tuberculosis drug isoniazid and its role in the protection of metal, Journal of Environmental Chemical Engineering, 2019, 7, 102971.
- M.A. Hegazy, H.M. Ahmed, A.S. El-Tabei, Investigation of the inhibitive effect of p-substituted 4-(N, N, N-dimethyldodecyl-ammonium bromide) benzylidene-benzene-2-yl-amine on corrosion of carbon steel pipelines in acidic medium, Corrosion Science, 2011, 53, 671–678.
- R. Hsissou, S. Abbout, A. Berisha, M. Berradi, M. Assouag, N. Hajjaji, A. Elharfi, Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution, Journal of Molecular Structure, 2019, 1182, 340–351.
- C. Gonzalez, H.B. Schlegel, Improved algorithms for reaction path following: Higher-order implicit algorithms, The Journal of Chemical Physics, 1991, 95, 5853–5860.
- C. Öğretir, B. Mihci, G. Bereket, Quantum chemical studies of some pyridine derivatives as corrosion inhibitors, Journal of Molecular Structure: Theochem., 1999, 488, 223–231.
- I. Lukovits, E. Kalman, F. Zucchi, Corrosion inhibitors—correlation between electronic structure and efficiency, Corrosion, 2001, 57, 3–8.
- S.K. Saha, A. Dutta, P. Ghosh, D. Sukul, P. Banerjee, Adsorption and corrosion inhibition effect of Schiff base molecules on the mild steel surface in 1 M HCl medium: a combined experimental and theoretical approach, Physical Chemistry Chemical Physics, 2015, 17, 5679–5690.
- R. Hsissou, S. Abbout, R. Seghiri, M. Rehioui, A. Berisha, H. Erramli, M. Assouag, A. Elharfi, Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations), Journal of Materials Research and Technology, 2020. https://doi.org/10.1016/j.jmrt.2020.01.002.
DOI: http://dx.doi.org/10.13171/mjc101020291189aa
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
