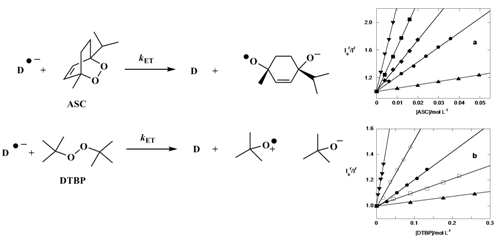
Kinetics of the photoinduced dissociative reduction of the model alkyl peroxides di-tert-butyl peroxide and ascaridole
Abstract
Full Text:
PDFReferences
- E. R. Gaillard and D. G. Whitten, Acc. Chem. Res., 1996, 29, 292.
- (a) F. D. Saeva, Top. Curr. Chem. 1990, 156, 61. (b) Saeva, F. D. in Advances in Electron Transfer Chemistry, Mariano, P. S. ed. JAI Press, New York, 1994, vol 4, p. 1; (c) X. Wang, F. D. Saeva and J. A. Kampmeier, J. Am. Chem. Soc., 1999, 121, 4364; (d) J. A.Kampmeier, A. K. M. Hoque, F. D. Saeva, D. K. Wedegaertner, P. Thomsen, S. Ullah, J. Krake, and T. Lund, J. Am. Chem. Soc., 2009, 131, 10015.
- (a) J. Bonin, C. Costentin, M. Mahet, J.-B. Mulon and Marc Robert, Phys. Chem. Chem. Phys., 2009, 11, 10275; (b) L. Pause, M. Robert and J.-M. Savéant, J .Am. Chem. Soc., 2000, 122, 9829; (c) S. M. Bonesi and R. Erra-Balsells, J. Chem. Soc. Perkin Trans. 2, 2000, 1583; (d) L. Chen, M. S. Farahat, H. Gan, S. Farid and D. G. Whitten, J. Am. Chem. Soc., 1995, 117, 6398; (e) L. Chen, M. S. Farahat, E. R. Gaillard, S. Farid and D. G. Whitten, J. Photochem. Photobio. A: Chem, 1996, 95, 21.
- (a) J. Mohanty, H. Pal, S.K. Nayak, S. Chattopadhyay and A.V. Sapre, Chem. Phys. Lett. 2003, 370, 641; (b) J. Mohanty, H. Pal and A. V. Sapre, J. Chem. Phys., 2002, 116, 8006; (c) J. Mohanty, H. Pal, S. K. Nayak, S. Chattopadhyay and A. V. Sapre, J. Chem. Phys., 2002,117, 10744; (d) S. Nath, A. K. Singh, D. K. Palit, A. V. Sapre and J. P. Mittal, J. Phys. Chem. A, 2001, 105, 7151; (e) S. Nath and A. V. Sapre, Chem. Phys. Lett. 2001, 344, 138.
- (a) J.-M. Savéant, in Advances in Physical Organic Chemistry, ed. T. T. Tidwell, Academic Press, New York, 2000, vol. 35, p. 117; (b) J.-M. Savéant, in Advances in Electron Transfer Chemistry, ed. P. S. Mariano, JAI Press, Greenwich, CT, 1994, vol 4, p. 53; (c) J.-M. Savéant, Acc. Chem. Res., 1993, 26, 455; (d) C. Costentin, M. Robert and J.-M. Savéant, Chem. Phys., 2006, 324, 40.
- (a) A. Houmam, Chem. Rev., 2008, 108, 2180; (b) R. A. Rossi, A. B. Pierini and A. B. Penenory, Chem. Rev., 2003, 103, 71.
- (a) L. Pause, M. Robert and J.-M. Savéant, J. Am. Chem. Soc., 2001, 123, 4886; (b) M. Robert and J.-M. Savéant, J. Am. Chem. Soc., 2000, 122, 514; (c) C. Costentin, M. Robert and J.-M. Savéant, J. Phys. Chem. A, 2000, 104, 7492; (d) L. Pause, M. Robert and J.-M. Savéant, ChemPhysChem, 2000, 1, 199.
- (a) R. L. Donkers, F. Maran, D. D. M. Wayner and M. S. Workentin, J. Am. Chem. Soc.,1999, 121, 7239; (b) M. S. Workentin, F. Maran and D. D. M. Wayner, J. Am. Chem. Soc.,1995, 117, 2120.
- (a) D. C. Magri and M. S. Workentin, Org. Biomol. Chem., 2003, 1, 3418; (b) S.Antonello,M. Musumeci, D. D. M. Wayner and F. Maran, J. Am. Chem. Soc., 1997, 119, 541.
- (a) R. L. Donkers and M. S. Workentin, Chem.ï€Eur. J., 2001, 7, 4012; (b) M. S. Workentin and R. L. Donkers, J. Am. Chem. Soc., 1998, 120, 2664.
- (a) D. L. B. Stringle, D. C. Magri and M. S. Workentin, Chem ï€Eur. J. 2010, 16, 178;(b) D. C. Magri and M. S. Workentin, Chem. ï€Eur. J. 2008, 14, 1698; (c) D. C. Magri and M. S. Workentin, Org. Biomol. Chem. 2008, 18, 3354; (d) F. Najjar, C. André-Barrès, C. Lacaze-Dufaure, D. C. Magri, M. S. Workentin and T. Tzèdakis, Chem.ï€Eur. J. 2007, 13, 1174; (e) R. L. Donkers and M. S. Workentin, J. Am. Chem. Soc. 2004, 126, 1688.
- (a) F. Polo, S. Antonello, F. Formaggio, C. Toniolo and F. Maran, J. Am. Chem. Soc.,2005, 127, 492; (b) S. Antonello, F. Formaggio, A. Moretto, C. Toniolo and F. Maran, J. Am. Chem. Soc., 2003, 125, 2874; (c) S. Antonello, M. Crisma, F. Formaggio, A. Moretto, F. Taddei, C. Toniolo and F. Maran, J. Am. Chem. Soc., 2002, 124, 11503; (d) S. Antonello, F.Formaggio, A. Moretto, C. Toniolo and F. Maran, J. Am. Chem. Soc. 2001, 123, 9577.
- (a) S. Antonello and F. Maran, J. Am. Chem. Soc., 1999, 121, 9668; (b) S. Antonello and F. Maran, J. Am. Chem. Soc., 1997, 119, 12595.
- (a) F. Maran, D. D. M. Wayner and M. S. Workentin, in Advances in Physical Organic Chemistry, ed. T. T. Tidwell and J. P. Richard, Academic Press, New York, 2001, vol 36, 85. (b) F. Maran and M. S. Workentin, Interface, 2002, 44.
- (a) M. V. Encinas, E. A. Lissi, J. Photochem. 1982, 20, 153; (b) P. S. Engel, T. L. Woods, M. A. Page, J. Phys. Chem. 1983, 87, 10; (c) J. C. Scaiano, G. G. Wubbels, J. Am. Chem. Soc. 1981, 103, 640.
- R. L. Donkers and M. S. Workentin, J. Phys. Chem. B. 1998, 102, 4061.
- D. C. Magri, R. L. Donkers and M. S. Workentin, J. Photochem. Photobiol. A. Chem., 2001, 138, 29.
- (a) K. Daasbjerg, S. U. Pederson and H. Lund, Acta Chem. Scand., 1991, 45, 424; (b) T. Lund and H. Lund, Acta Chem. Scand. B, 1986, 40, 470; (c) C. P. Andrieux, I. Gallardo, J.-M. Savéant and K.-B. Su, J. Am. Chem. Soc., 1986, 108, 638; (d) S. Fukuzumi, S. Kuroda, and T. Tanaka, J. Chem. Soc. Perkin Trans. 2, 1986, 25.
- (a) J. Grimshaw, J. R. Langan and G. A. Salmon, J. Chem. Soc. Faraday. Trans., 1994, 90, 75; (b) J. Grimshaw, J. R. Langan and G. A. Salmon, J. Chem. Soc. Chem. Comm., 1988, 1115.
- N. Sutin, Acc. Chem. Res., 1982, 15, 275.
- (a) J.-M. Savéant, J. Am. Chem. Soc., 1987, 109, 6788; (b) J.-M. Savéant, J. Am. Chem. Soc., 1992, 114, 10595.
- (a) P. Suppan, J. Chem. Soc. Faraday Trans., 1986, 82, 509; (b) E. Vauthey, P. Suppan, and E. Haselbach, Helv. Chim. Acta., 1986, 69, 430. Suppan proposed that any screening between the two point charges results only from the polarizability of the solute molecules such that the Coulomb term is no longer solvent dependent, in which case, a modified Coulomb term is applicable where n is the refractive index and rDA is the combined effective radius of donor and acceptor: –q2/2n2rDA. We tested the term using the radii of ASC and DTBP determined from their density, ï², using the equation rAB(Ã…)=108[(3M/4ï°Nï²)1/3], where M is the molar mass, N is Avogrado's number and converted it to an effective radius.9,10 The calculated radii of ASC and DTBP are 2.7 and 2.8 Ã…, respectively. A van-der Waals' radius of 1.9 Ã… was used for the donors, which is half the thickness of a planar ï° system providing a centre-to-centre distance between the sensitizer and ASC and DTBP of 4.6 Ã… and 4.7 Ã…, respectively. The calculated average contribution from the modified Coulomb term is –18 kcal mol-1, which when applied to the photochemical kinetic data results in an excellent parabolic relationship. This result is likely fortuitous as the validity of a modified Coulomb term has been scrutinised: See M. Tachiya, Chem. Phys. Lett., 1994, 230, 491.
- (a) A. Cardinale, A. A. Isse, A. Gennaro, M. Robert, and J.-M. Savéant, J. Am. Chem. Soc., 2002, 124, 13533; (b) C. Costentin, P. Hapiot, M. Médebielle, and J.-M. Savéant, J. Am. Chem. Soc., 2000, 122, 5623.
- A. Houmam and E. M. Hamed, Phys. Chem. Chem. Phys. 2012, 14, 113.
- J. F. Callan, A. P. de Silva and N. D. McClenaghan, Chem. Commun. 2004, 2048.
- Although photoinduced DET is thermodynamically feasible for both ASC and DTBP, other processes may compete, which can result in quantum yields for photoinduced DET less than unity as reported for alkyl halides. See references 3b and 7.
- L. Eberson, M. Ekström, T. Lund and H. Lund, Acta. Chem. Scand. 1989, 43, 101. 28 - G. O. Schenck, Agnew. Chem., 1952, 64, 12.
DOI: http://dx.doi.org/10.13171/mjc.1.6.2012.10.06.13
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
