Global reactivity Indices and Electron Localization Function calculations in the formation of boron nitride molecule
Abstract
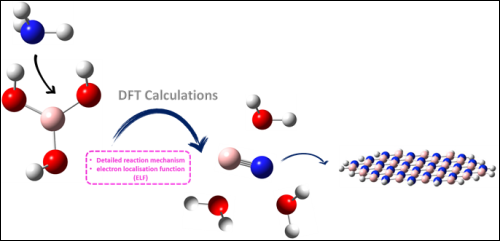
The present study focuses on topological analysis of electron density population, structural and thermodynamic properties involved in the reaction between Boric Acid (H3BO3) and Ammonia (NH3) in the synthesis of boron nitride (BN) used in cancer therapy medication and biomedical applications. The compound above has similar properties to carbonaceous materials. Indeed, it exists mainly in the two cubic and hexagonal forms, which are respectively identical to diamond and graphene surfaces. DFT/ M06-2X/aug-cc-pVDZ calculations were performed to determine global reactivity indices and the reaction process that operates via three transition states. ELF function has been achieved to describe the evolution of forming bonds along the IRC path.
Full Text:
PDFReferences
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara, D. Golberg, Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties, Adv Mater., 2009, 21, 2889–2893.
- J. Taha-Tijerina, T. N. Narayanan, G. Gao, M. Rohde, D. A. Tsentalovich, M. Pasquali, P. M. Ajayan, Electrically Insulating Thermal Nano-Oils Using 2D Fillers, ACS Nano., 2012, 6, 1214–1220.
- H. Zhu, Y. Li, Z. Fang, J. Xu, F. Cao, J. Wan, C. Preston, B. Yang, L. Hu, Highly Thermally Conductive Papers with Percolative Layered Boron Nitride Nanosheets, ACS Nano., 2014, 8, 3606–3613.
- L. H. Li, J. Cervenka, K. Watanabe, T. Taniguchi, Y. Chen, Strong Oxidation Resistance of Atomically Thin Boron Nitride Nanosheets, ACS Nano., 2014, 8, 1457–1462.
- Z. Liu, Y. Gong, W. Zhou, L. Ma, J. Yu, J. C. Idrobo, J. Jung, A. H. MacDonald, R. Vajtai, J. Lou, P. M. Ajayan, Ultrathin high-temperature oxidation-resistant coatings of hexagonal boron nitride, Nat Commun., 2013, 4, 2541.
- B. Singh, G. Kaur, P. Singh, K. Singh, B. Kumar, A. Vij, M. Kumar, R. Bala, R. Meena, A. Singh, A. Thakur, A. Kumar, Nanostructured Boron Nitride with High Water Dispersibility For Boron Neutron Capture Therapy, Sci Rep., 2016, 6, 35535.
- W. Koch, M. C. Holthausen, A Chemist's Guide to Density Functional Theory, 2nd, 2001.
- R. G. Parr, Density Functional Theory of Atoms and Molecules, Horizons of Quantum Chemistry, Springer Netherlands: Dordrecht, 1980, 5–15.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A.Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts,
B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada,M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro,
M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma,
O. Farkas, J. B. Foresman, D. J. Fox, Gaussian 09, Gaussian Inc, Wallingford CT, 2009.
-Y. Zhao, D. G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor Chem Account., 2008, 120, 215–241.
- T. H. Dunning, Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen, J Chem Phys., 1989, 90, 1007–1023.
- R. Dennington, T.A. Keith, J.M. Millam, Gauss view, Version 5, Semichem. Inc., Shawnee Mission, 2009.
- C. Gonzalez, H.B. Schlegel. An improved algorithm for reaction path following, J. Chem.Phys., 1989, 9, 2154-2161.
- S. Saha, R. K. Roy, S. Pal, CDASE-A reliable scheme to explain the reactivity sequence between Diels–Alder pairs, Phys Chem Phys., 2010, 12, 9328.
- P. Geerlings, F. De Proft, W. Langenaeker, Conceptual Density Functional Theory, Chem Rev., 2003, 103, 1793–1874.
- T. Joseph, H. T. Varghese, C. Y. Panicker,K. Viswanathan, M. Dolezal, C. Van Alsenoy, Spectroscopic (FT-IR, FT-Raman), first-order hyperpolarizability, NBO analysis, HOMO and LUMO analysis of N-[(4-(trifluoromethyl)phenyl]pyrazine-2-carboxamide by density functional methods, Arabian Journal of Chemistry, 2017, 10, S2281–S2294.
- Z. Hasanzade, H. Raissi, Solvent/co-solvent effects on the electronic properties and adsorption mechanism of anticancer drug Thioguanine on Graphene oxide surface as a nanocarrier: Density functional theory investigation and a molecular dynamic, Applied Surface Science, 2017, 422, 1030–1041.
- E. D. Glendening, A. E. Reed, J. E. Carpenter, F. Weinhold, NBO Programm, Version 1.1.
- H. El Hadki, F. Hlimi, M. Salah, K. Marakchi, N. Komiha, O. K. Kabbaj, Theoretical Study of Reaction Between Nitrilimine and 1,4 Oxazine 2 Carboxylate by MP2 and DFT Methods, Oriental Journal of Chemistry, 2018, 34, 2992–2997.
- R. Tazi, H. E. Hadki, M. Salah, A. Zrineh, M. E. Azzouzi, N. Komiha, Theoretical Approach of the Adsorption of Herbicide Amitrole on the Soil using DFT Method, Oriental Journal of Chemistry, 2018, 34, 1240–1248.
DOI: http://dx.doi.org/10.13171/mjc107020071343aeh
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
