Synthesis of 8-Hydroxyquinoline/exchanged montmorillonite hybrids: Sorption, Luminescence and Thermal stability studies
Abstract
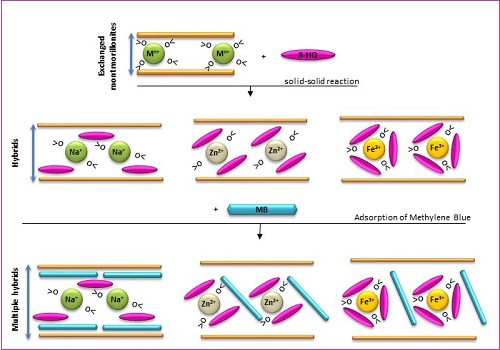
Hybrids (H) prepared from 8-Hydroxyquinoline (8-HQ, "oxine") and exchanged montmorillonites (Na(I)-, Zn(II)- and Fe(III)-Mont) have been synthesized using solid-solid reactions at room temperature. The characterization of these hybrids by PPXRD has shown that the interlayer spaces swell (from 0.22 to 1.10 Å of d001 differences) after the inclusion of 8-HQ due to its complexation with the cations present. In the IR spectra, new bands corresponding to 8-HQ groups, appear in the regions 1244 - 1608 cm-1 and 574 - 826 cm-1. DSC measurement has shown the hybrids to be more stable than the precursor montmorillonites, 8-HQ and the simple metal oxinates. Zeta potential measurement on suspensions of the hybrids showed them to be negatively charged over the whole pH range (pH=2-10). As an application of our elaborated hybrids, sorption of the cationic dye methylene blue MB (CMB=200 mg.L-1) by the Na(I)-, Zn(II)- and Fe(III)-hybrids has been found to be rapid for the first two. At the same time, for the Fe(III) species, one month of sedimentation was required to adsorb 87 % of MB. The structural characterization of multi-hybrids MH (H+MB) by PXRD has shown a shift of d001 to higher values (Na-MH: 15.32 Å; Zn-MH: 16.61 Å; Fe-MH: 16.99 Å), signifying the intercalation of MB into interlayer spaces.
Full Text:
PDFReferences
- R. Zhu, Q. Zhou, J. Zhu, Y. Xi, H. He, Organo-Clays As Sorbents of Hydrophobic Organic Contaminants: Sorptive Characteristics and Approaches to Enhancing Sorption Capacity, Clays Clay Miner., 2015, 63, 199. https://doi.org/10.1346/CCMN.2015.0630304.
- Q. Wang, Y. Wang, L. Chen, A green composite hydrogel based on cellulose and clay as an efficient absorbent of colored organic effluent, Carbohydrate Polymers, 2019, 210, 314-321.
https://doi.org/10.1016/j.carbpol.2019.01.080.
- A. A. Adeyemo, I. O. Adeoye, O. S. Bello, Adsorption of dyes using different types of clay: a review, Appl Water Sci., 2017, 7, 543–568. https://doi.org/10.1007/s13201-015-0322-y.
- F. Ayari, E. Srasra, M. Trabelsi-Ayadi, Retention of organic molecule quinalizarin" by bentonitic clay saturated with different cations, Desalination, 2007, 20, 499-506. https://doi.org/10.1016/j.desal.2006.03.578.
- M. Lackovičová, T. Baranyaiová, J. Bujdák, The chemical stabilization of methylene blue in colloidal dispersions of smectites, Applied Clay Sciences, 2019, 181, 105222. https://doi.org/10.1016/j.clay.2019.105222.
- T. S. Anirudhan, M. Ramachandran, Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm, Proces. Saf. Env. Protect., 2015, 95, 215-225. https://doi.org/10.1016/j.psep.2015.03.003.
- A. H. Jawad, A. S. Abdulhameed, Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study, Surfaces and Interfaces, 2020, 18, 100422. https://doi.org/10.1016/j.surfin.2019.100422.
- A. S. Abdulhameed, A. T. Mohammad, A. H. Jawad, Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye, Journal of Cleaner Production, 2019, 232, 43-56. https://doi.org/10.1016/j.jclepro.2019.05.291.
- A. S. Abdulhameed, A. T. Mohammad, A. H. Jawad, Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/TiO2 nanocomposite: Application of response surface methodology, Desalination and Water Treatment, 2019, 164 346–360. https://doi.org/10.5004/dwt.2019.24384.
- F. Ayari, M. T. Ayadi, Inorganic and organic smectite for synthetic and real textile water treatment. Optical and luminescence properties, Desalination and Water Treatment, 2018, 125, 47-60. https://doi.org/10.5004/dwt.2018.22696.
- B. Erdem, A. Özcan, Adsorption and solid-phase extraction of 8-hydroxyquinoline from aqueous solutions by using natural bentonite, Appl. Surf. Sci., 2010, 256, 5422–5427. https://doi.org/10.1016/j.apsusc.2009.12.126.
- B. Mellah, D. Sall, I. Msaddak, E. Srasra, Intercalation of 8-hydroxyquinoline into Na(I)- and Zn(II)-Tunisian montmorillonites: characterization and luminescence properties of elaborated hybrids, J. Inclus. Phenom. Macrocycl. Chem., 2019, 94, 309-318. https://doi.org/10.1007/s10847-018-0826-9.
- N. Khaorapaponga, M. Ogawa, In situ formation of bis(8-hydroxyquinoline) zinc(II) complex in the interlayer spaces of smectites by solid–solid reactions, Journal of Physics and Chemistry of Solids , 2008, 69, 941–948. https://doi.org/10.1016/j.jpcs.2007.10.092.
- N. Khaorapapong, M. Ogawa, Solid-state intercalation of 8-Hydroxyquinoline into Li(I)-, Zn(II)- and Mn(II)-montmorillonites, Applied Clay Science, 2007, 35, 31-38. https://doi.org/10.1016/j.clay.2006.08.003.
- N. Khaorapapong, K. Kuroda, M. Ogawa, Intercalation of 8-hydroxyquinoline into Al-smectites by solid-solid reactions, Clays and Clay Minerals, 2002, 50, 428-434. https://doi.org/10.1346/000986002320514154.
- P. Pimchan, N. Khaorapapong, M. Ogawa, Preparation of a series of group XIII metal–quinolate complexes in natural and synthetic smectites, Applied Clay Science, 2011, 54, 3–4, 287-291, https://doi.org/10.1016/j.clay.2011.09.002.
- F. Bergaya, G. Lagaly, General introduction: clays, clay minerals, and clay science, Dev. Clay Sci., 2006, 1, 1–18. https://doi.org/10.1016/S1572-4352(05)01001-9.
- J. Manjanna, T. Kozaki, S. Sato, Fe(III)-montmorillonite: Basic properties and diffusion of tracers relevant to alteration of bentonite in deep geological disposal, Applied Clay Science, 2009, 43, 208-217. https://doi.org/10.1016/j.clay.2008.09.007.
- J. Bishop, J. Madejová, P. Komadel, H. Fröschl, The influence of structural Fe, Al and Mg on the infrared OH bands in spectra of dioctahedral smectites, Clay Minerals, 2002, 37, 607-616. https://doi.org/10.1180/0009855023740063.
- Y. E. Dolaksiz, F. Temel, M. Tabakci, Adsorption of phenolic compounds onto calix[4]arene-bonded silica gels from aqueous solutions, Reactive and Functional Polymers, 2018, 126, 27-35. https://doi.org/10.1016/j.reactfunctpolym.2018.03.003.
- L. Lynne, J. R. Merrit, R. T. Cady, B. W. Miundy, The Crystal Structure of Zinc 8-Hydroxyquinolinate Dihydrate, ActaCrystallogr., 1954, 7, 473. https://doi.org/10.1107/S0365110X54001491.
- I. A. Pastre, D. N. Oliveira, A. B. S. Moitinho, G. R. de Souza, E. Y. Ionashiro, F. L. Fertonani, Thermal behavior of intercalated 8-hydroxyquinoline (oxine) in montmorillonite clay, J. Therm. Analy. Calom., 2004, 75, 661–669. https://doi.org/10.1023/B:JTAN.0000027160.19388.0c.
- B. T. Franzin, C. P. Lupi, L. A. Martins, F. C. Guizellini, C. C. Marques dos Santos, I. A. Pastre, F. L. Fertonani, Thermal and electrochemical studies of Fe(III) organophilic montmorillonite, J. Therm. Anal. Calorim., 2018, 131, 713-723. https://doi.org/10.1007/s10973-017-6327-z.
- D. Singh, V. Nishal, S. Bhagwan, R. K. Saini, I. Singh, Electroluminescent materials: Metal complexes of 8-hydroxyquinoline - A review." Materials & Design, 2018, 156, 215-228. https://doi.org/10.1016/j.matdes.2018.06.036.
- C. Schmidt, H. W. Thelakkat, Lithium−Quinolate complexes as emitter and interface materials in organic light-emitting diodes, Chem Mater., 2000, 12, 3012-19. https://doi.org/10.1021/cm0010248.
- F. Dumur, L. Beouch, M. A. Tehfe, E. Contal, M. Lepeltier, G. Wantz, B. Graff, F. Goubard, C. R. Mayer, J. Lalevée, D. Gigmes, Low-cost zinc complexes for white organic light-emitting devices, Thin Solid Films, 2014, 564, 351-60. https://doi.org/10.1016/j.tsf.2014.06.006.
- L. S. Sapochak, E. F. Benincasa, R. S. Schofield, J. L. Baker, K. K. C. Riccio, D. Fogarty, H. Kohlmann, K. F. Ferris, P. E. Burrows, Electroluminescent zinc(II) bis(8-hydroxyquinoline): structural effects on electronic states and device performance, J. Am.Chem. Soc., 2002, 124, 6119-6125. https://doi.org/10.1021/ja0201909.
- M. Vinuth, H. S. B. Naik, M. M. Mahadevaswamy, M. C. Prabhakara, Environmentally benign Fe(III)–montmorillonite for rapid adsorption of methylene blue dye in aqueous medium under ambient conditions, Fash. Text., 2017, 4, 8. https://doi.org/10.1186/s40691-017-0087-z.
- H. Shao, N. Cao, H. Xiao, Adsorption properties of dyeing wastewater on 8-hydroxyquinoline modified bentonite", Applied Mechanics and Materials, 2011, 71-78 1118-1122. https://doi.org/10.4028/www.scientific.net/AMM.71-78.1118.
- F. Temel, M. Turkyilmaz, S. Kucukcongar, Removal of methylene blue from aqueous solutions by silica gel supported calix[4]arene cage: Investigation of adsorption properties, European Polymer Journal, 2020, 125, 109540. https://doi.org/10.1016/j.eurpolymj.2020.109540.
- Q. Han, J. Wang, B. A. Goodman, J. Xie, Z. Liu, High adsorption of methylene blue by activated carbon prepared from phosphoric acid-treated eucalyptus residue, Powder Technology, 2020, 366, 239-248. https://doi.org/10.1016/j.powtec.2020.02.013.
DOI: http://dx.doi.org/10.13171/mjc10502005201345bm
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
