Original process for Thymol Para-sulfonation: A Preliminary Optimization Study
Abstract
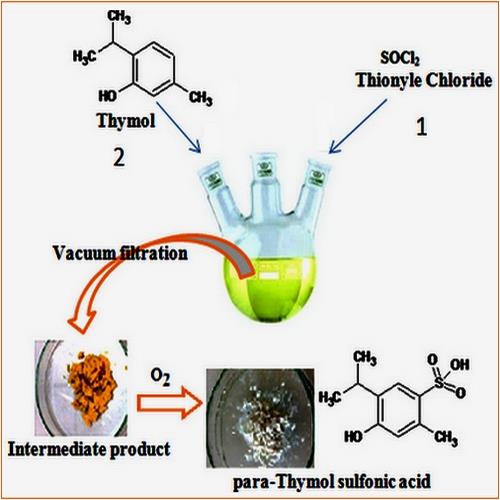
In this study, we described an original, new, simple, and productive one-pot method for Thymol para-sulfonation by using thionyl chloride (SOCl2) as a sulfonating agent under mild conditions. Spectroscopic analysis by FT-IR, mass spectrometry, and NMR 1H and 13C confirmed the structure of the parasulfonated derivative of Thymol: the 4-hydroxy-5-isopropyl-2-methylbenzenesulfonic acid. An optimization study was carried out to perform and improve the yield of this new para-sulfonation method by studying the effect of three parameters: temperature, the molar ratio (η) of the reactants, and solvents. The obtained results showed that the use of cyclohexane as a solvent, a molar ratio greater or equal than 3, and working under low temperatures increase the yield of this parasulfonation considerably to reach adequately 91.3%.
Full Text:
PDFReferences
- L. Jiang, L. Gao, Y. Liu, Adsorption of salicylic acid, 5-sulfosalicylic acid, and Tiron at the alumina–water interface, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 211, 165-172.
- G. Smith, U.D. Wermuth, D.J. Young, J.M. White, Polymeric structures in the metal complexes of 5-sulfosalicylic acid: The rubidium(I), cesium (I) and lead(II) analogs, Polyhedron, 2007, 26, 3645-3652.
- Y. Shudo, M.R. Karim, R. Ohtani, M. Nakamura, S. Hayami, Hybrids from the π−π Stacking of Graphene Oxide and Aromatic Sulfonic Compounds for Improved Proton Conductivity, ChemElectroChem, 2017, 5, 238-241.
- F. Kucera, J. Jancfi, Homogeneous and heterogeneous sulfonation of polymers: a review, Polymer Engineering & Science, 1998, 38, 783-792.
- O. Lindner, L. Rodefeld, Benzenesulfonic Acids, and their Derivatives, Encyclopedia of Industrial Chemistry, 2000, 5, 259-306.
- E. Gilbert, Recent Developments in Preparative Sulfonation and Sulfation, Synthesis, 1969, 01, 3-10.
- D. Daoust, J. Devaux, P. Godard, Mechanism and kinetics of poly (ether ether ketone) (PEEK) sulfonation in concentrated sulfuric acid at room temperature Part 1. Qualitative comparison between, Polymer International, 2001, 50, 917-924.
- M. Puertas-Mejía, S. Hillebrand, E. Stashenko, P. Winterhalter, In-vitro radical scavenging activity of essential oils from Columbian plants and fractions from oregano (Origanum vulgare L) essential oil, Flavour, and Fragrance Journal, 2002, 17, 380–384.
- L.R. Salgueiro, E, Pinto, M.J. Gonçalves, C. Pina-Vaz, C. Cavaleiro, A.G. Rodrigues, A. Palmeira, Chemical composition and antifungal activity of the essential oil of Thymbra capitata, Planta Med. 2004, 70, 572-575.
- T. Yoshida, K. Mori, G.X. He, Inulavosin, a new thymol dimer with piscicidal activity from Inula Nervosa, Heterocycles, 1995, 41, 1923-1926.
- F. Oke, B. Aslim, S. Oztirk, Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten, Altundag S, Food Chem. 2009, 112, 874-879.
- C.C. Liolios, O. Gortzi, S. Lalas., J. Tsaknis, I. Chinou, Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity, Food Chem. 2009, 112, 77-83.
- N. Dirdy, L. Dubreuil, M. Pinkas, Antibacterial activity of thymol, carvacrol and cinnamaldehyde alone or in combination, Pharmazie, 1993, 48, 301-304.
- M.G. Botelho, The minimum inhibitory concentration of oral antibacterial agents against cariogenic organisms, Microbios, 2000, 103, 31-41.
- P. L. Teissedre, A. Waterhouse, L, Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties, Journal of Agricultural and Food Chemistry, 2000, 48, 3801–3805.
- D.D. Jyoti, D. Singh, G. Kumar, M. Karnatak, S. Chandra, V.V. Prakash, R. Shankar, Thymol Chemistry: A Medicinal Toolbox, Current Bioactive Compounds, 2019, 15, 454-474.
- S. Liu, X. Liu, C. Wang, Isopropylation of m-Cresol Catalyzed by Recoverable Acidic Ionic liquids, Industrial & Engineering Chemistry Research, 2013, 52, 16719-16723.
- M. Selvaraj, S. Kawi, Comparison of mesoporous and microporous solid acid catalysts for highly selective synthesis of thymol by vapor phase isopropylation of m-cresol, Microporous and Mesoporous Materials, 2008, 109, 458-469.
- R. Amandi, J.R. Hyde, S.K. Ross, T.J. Lotz, M. Poliakoff, Continuous reactions in supercritical fluids; a cleaner, more selective synthesis of thymol in supercritical CO2, Green Chemistry, 2005, 7, 288-293.
- V. Umamaheswari, M. Palanichamy, V. Murugesan, Isopropylation of m-Cresol over Mesoporous Al–MCM-41 Molecular Sieves, Journal of Catalysis, 2002, 210, 367–374.
- H. Grabowska, W. Miśta, J. Trawczyński, J. Wrzyszcz, M. Zawadzki, A method for obtaining thymol by gas phase catalytic alkylation of m-cresol over zinc aluminate spinel, Applied Catalysis A General, 2001, 220, 207–213.
- S. Velu, S. Sivasanker, Alkylation of m-cresol with methanol and 2-propanol over calcined magnesium-aluminum hydrotalcite, Research on Chemical Intermediates, 1998, 24, 657–666.
- C.T. O'Connor, G. Moon, W. Böhringer, J.C.Q. Fletcher, Alkylation of Phenol and m-Cresol Over Zeolites, Collection of Czechoslovak Chemical Communications, 2003, 68, 1949–1968.
- M. Nitta, K. Yamaguchi, K. Aomura, The Alkylation of m-Cresol with Propylene by Supported Metal Sulfates, Bulletin of the Chemical Society of Japan, 1974 , 47, 2897–2898.
- P. Wimmer, H.J. Buysch, L. Puppe, Process for the preparation of thymol, United States Patent N° 5,030,770, 1991.
- P. Max, Manufacture of synthetic thymol, United States Patent N°1,432,298, 1922.
- D.R. Stevens, Process for producing substituted hydroxy aromatic compounds, United States Patent N° 2,603, 662, 1952.
- R. R Bottoms, Crestwood, Production of Thymol, United States Patent N° 2, 840, 616, 1958.
- N. Abbassi, H. Chicha, E. M. Rakib, A. Hannioui, M. Alaoui, A. Hajjaji M. Viale, Synthesis, antiproliferative and apoptotic activities of N-(6(4)-indazolyl)-benzenesulfonamide derivatives as potential anticancer agents, European Journal of Medicinal Chemistry, 2012, 57, 240–249.
- B. Oulemda, E. M. Rakib, N. Abbassi, M. Saadi, L. El Ammari, 1-(4-Methylphenylsulfonyl)-5,6-dinitro-1H-indazole, Acta Crystallographica Section E Structure Reports Online, 2013, 70, 101.
https://doi.org/10.1107/S1600536813034326.
- M. Xiaoke, Li. Peng, M. Tian-Xiang, Guang-Wen Chu, Yong Luo, Hai-Kui Zou, Study on phenol sulfonation by concentrated sulfuric acid: Kinetics and process optimization, Chemical Engineering Science, 2019, 202, 15-25.
- A. Abdolmaleki, M. Zhiani, M. Maleki, S. Borandeh, K. Firouz, Preparation and evaluation of sulfonated polyoxadiazole membrane containing phenol moiety for PEMFC application, Polymer, 2015, 75, 17-24.
DOI: http://dx.doi.org/10.13171/mjc10602006191398as
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
