Biosorption of Copper (Cu 2+) in aqueous solution by an adsorbent materiel prepared from marine algae (Bifurcaria bifurcata): Equilibrium and kinetic studies
Abstract
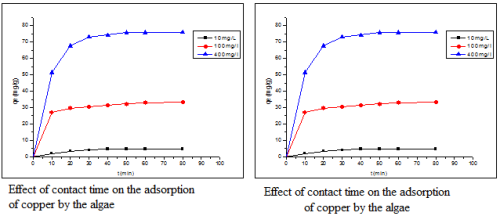
Morocco is a country influenced by the 3500 km of coastline, with the presence of many marine species. Among these species, there is a wide variety of marine algae that have been specifically used in pharmacological studies, but also environmental. Among these seaweed species Bifurcaria bifurcata having a very large biomass was used as a novel adsorbent for the removal of copper in aqueous solution. Adsorption tests showed that the equilibrium is established after 60 minutes. Various experimental parameters were analyzed: pH, adsorbent mass and initial concentration of copper. Experimental results have shown that the adsorption of copper on seaweed pH dependent of the solution and the initial concentration of copper.
The adsorption capacity was determined using the Langmuir and Freundlich isotherms. The maximum adsorption capacity is 101.9 mg.g-1. The kinetic study of the adsorption of copper on the algae showed that the adsorption process follows the pseudo-second order kinetic model with high R2 values.
Â
Â
Full Text:
PDFDOI: http://dx.doi.org/10.13171/mjc.4.2.2015.08.04.11.19/khamliche
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
