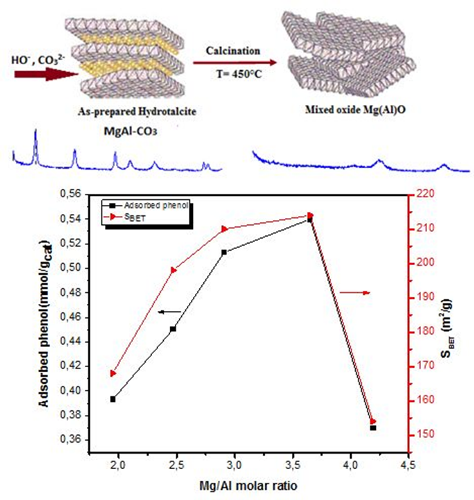
Effect of Mg/Al molar ratio on the basicity of Mg-Al mixed oxide derived from Mg-Al hydrotalcite
Abstract
The fundamental character of the Mg-Al mixed oxide (Mgn(Al)O), derived from the Mg-Al hydrotalcite (MgnAl-CO3-HT), where n corresponds to the Mg/Al molar ratio (n: 2, 2.5, 3, 3.5 and 4), was studied by using the adsorption of phenol as a probe acid molecule. The hydrotalcite precursors were prepared by the coprecipitation method. Their derived mixed oxides were obtained by thermal treatment at 450°C in a flow of air. The resulting solids were characterized by X-ray fluorescence spectroscopy (XRF), X-ray diffraction (XRD), infrared spectroscopy (FTIR), thermogravimetric and differential thermal analysis (TG-DTA), nitrogen physisorption (BET) and phenol chemisorption. The phenol adsorption followed by UV-Visible spectrophotometry shows that the basicity increases with the Mg/Al molar ratio, such that maximum quantity of phenol adsorbed (Qads = 0.54 mmol/g cat) was obtained with the mixed oxide derived from the Mg-Al hydrotalcite of Mg/Al molar ratio equal to 3.5.
Full Text:
PDFReferences
- S. Miyata, The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: the Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−, Clays Clay Miner, 1975, 23, 369–375.
- F. Cavani, F. Trifiro, A. Vaccari, Hydrotalcite-type anionic clays: Preparation, properties and applications, Catal today, 1991, 11, 173–301.
- A. Trave, A. Selloni, A. Goursot, D. Tichit, J. Weber, First-principles study of the structure and chemistry of Mg-based hydrotalcite-like anionic clays, J Phys Chem B, 2002, 106, 12291–12296.
- A. De Roy, C. Forano, K. El Malki, J.-P. Besse, Anionic clays: trends in pillaring chemistry, Expanded Clays and other microporous solids, Springer, 1992, 108–169.
- P. Kuśtrowski, L. Chmielarz, E. Bożek, M. Sawalha, F. Roessner, Acidity and basicity of hydrotalcite derived mixed Mg-Al oxides studied by test reaction of MBOH conversion and temperature-programmed desorption of NH3 and CO2, Mater Res Bull, 2004, 39, 263–281.
- D. Tichit, M. H. Lhouty, A. Guida, B. H. Chiche, F. Figueras, A. Auroux, D. Bartalini, E. Garrone, Textural properties and catalytic activity of hydrotalcites, J Catal, 1995, 151, 50–59.
- O. D. Pavel, D. Tichit, I.-C. Marcu, Acido-basic and catalytic properties of transition-metal containing Mg-Al hydrotalcites and their corresponding mixed oxides, Appl Clay Sci, 2012, 61, 52–58.
- J. I. Di Cosimo, V. K. Dıez, M. Xu, E. Iglesia, C. R. Apesteguıa, Structure and surface and catalytic properties of Mg-Al basic oxides,
J Catal, 1998, 178, 499–510.
- M. Di Serio, M. Ledda, M. Cozzolino, G. Minutillo, R. Tesser, E. Santacesaria, Transesterification of soybean oil to biodiesel by using heterogeneous basic catalysts, Ind Eng Chem Res, 2006, 45, 3009–3014.
- W. Xie, H. Peng, L. Chen, Calcined Mg-Al hydrotalcites as solid base catalysts for methanolysis of soybean oil, J Mol Catal A Chem, 2006, 246, 24–32.
- Y. Xi, R. J. Davis, Influence of textural properties and trace water on the reactivity and deactivation of reconstructed layered hydroxide catalysts for transesterification of tributyrin with methanol, J Catal, 2009, 268, 307–317.
- M. J. Climent, A. Corma, S. Iborra, K. Epping, A. Velty, Increasing the basicity and catalytic activity of hydrotalcites by different synthesis procedures, J Catal, 2004, 225, 316–326.
- J. Pérez-Ramírez, A. Ribera, F. Kapteijn, E. Coronado, C. J. Gómez-García, Magnetic properties of Co–Al, Ni–Al, and Mg–Al hydrotalcites and the oxides formed upon their thermal decomposition, J Mater Chem, 2002, 12, 2370–2375.
- U. Holzwarth, N. Gibson, The Scherrer equation versus the debye-Scherrer equation’, Nat Nanotechnol, 2011, 6, 534.
- S. K. Yun, T. J. Pinnavaia, Water content and particle texture of synthetic hydrotalcite-like layered double hydroxides, Chem Mater, 1995, 7, 348–354.
- K. K. Rao, M. Gravelle, J. S. Valente, F. Figueras, Activation of Mg–Al hydrotalcite catalysts for aldol condensation reactions, J Catal, 1998, 173, 115–121.
- P. Kuśtrowski, D. Sułkowska, L. Chmielarz, A. Rafalska-Łasocha, B. Dudek, R. Dziembaj, Influence of thermal treatment conditions on the activity of hydrotalcite-derived Mg-Al oxides in the aldol condensation of acetone, Microporous Mesoporous Mater, 2005, 78, 11–22.
- S. Abelló, F. Medina, D. Tichit, J. Pérez‐Ramírez, J. C. Groen, J. E. Sueiras, P. Salagre, Y. Cesteros, Aldol condensations over reconstructed Mg-Al hydrotalcites: structure-activity relationships related to the rehydration method, Chem Eur J, 2005, 11, 728–739.
- S. K. Sharma, P. K. Kushwaha, V. K. Srivastava, S. D. Bhatt, R. V Jasra, Effect of hydrothermal conditions on structural and textural properties of synthetic hydrotalcites of varying Mg/Al ratio, Ind Eng Chem Res, 2007, 46, 4856–4865.
- D. L. Bish, G. W. Brindley, A reinvestigation of takovite, a nickel aluminum hydroxy-carbonate of the pyroaurite group, Am Mineral, 1977, 62, 458–464.
- J. T. Kloprogge, D. Wharton, L. Hickey, R. L. Frost, Infrared and Raman study of interlayer anions CO32−, NO3−, SO42− and ClO4− in Mg/Al-hydrotalcite, Am Mineral, 2002, 87, 623–629.
- M. K. Titulaer, J. B. H. Jansen, J. W. Geus, The quantity of reduced nickel in synthetic takovite: effects of preparation conditions and calcination temperature, Clays Clay Miner, 1994, 42, 249–258.
- M. Del Arco, P. Malet, R. Trujillano, V. Rives, Synthesis and characterization of hydrotalcites containing Ni (II) and Fe (III) and their calcination products, Chem Mater, 1999, 11, 624–633.
- A. Morato, C. Alonso, F. Medina, Y. Cesteros, P. Salagre, J. E. Sueiras, D. Tichit, B. Coq, Palladium hydrotalcites as precursors for the catalytic hydroconversion of CCl2F2 (CFC-12) and CHClF2 (HCFC-22), Appl Catal B Environ, 2001, 32, 167–179.
- F. Prinetto, G. Ghiotti, P. Graffin, D. Tichit, Synthesis and characterization of sol-gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples, Microporous Mesoporous Mater, 2000, 39, 229–247.
- W. T. Reichle, S. Y. Kang, D. S. Everhardt, The nature of the thermal decomposition of a catalytically active anionic clay mineral, J Catal, 1986, 101, 352–359.
- T. Hibino, Y. Yamashita, K. Kosuge, A. Tsunashima, Decarbonation behavior of Mg-Al-CO3 hydrotalcite-like compounds during heat treatment, Clays Clay Miner, 1995, 43, 427–432.
- R. L. Frost, W. N. Martens, K. L. Erickson, Thermal decomposition of the hydrotalcite: Thermogravimetric analysis and hot stage Raman spectroscopic study, J Therm Anal Calorim, 2005, 82, 603–608.
- G. Leofanti, M. Padovan, G. Tozzola, B. Venturelli, Surface area and pore texture of catalysts, Catal today, 1998, 41, 207–219.
- D. Brunauer, W. E. LSD, Deming, and Teller, J Am Chem Soc, 1940, 62, 1723.
- M. Thommes, K. Kaneko, A. V Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K. S. W. Sing, Physisorption of gases, with special reference to the evaluation of the surface area and pore size distribution (IUPAC Technical Report), Pure Appl Chem, 2015, 87, 1051–1069.
- F. Li, X. Jiang, D. G. Evans, X. Duan, Structure and basicity of mesoporous materials from Mg/Al/In layered double hydroxides prepared by separate nucleation and aging steps method, J Porous Mater, 2005, 12, 55–63.
- K. Parida, J. Das, Mg/Al hydrotalcites: preparation, characterization, and ketonization of acetic acid, J Mol Catal A Chem, 2000, 151, 185–192.
- A. Corma, S. Iborra, S. Miquel, J. Primo, Catalysts for the production of fine chemicals: production of food emulsifiers, monoglycerides, by glycerolysis of fats with solid base catalysts,
J Catal, 1998, 173, 315–321.
- M. J. Climent, A. Corma, S. Iborra, J. Primo, Base catalysis for fine chemicals production: Claisen-Schmidt condensation on zeolites and hydrotalcites for the production of chalcones and flavanones of pharmaceutical interest, J Catal, 1995, 151, 60–66.
- T. Nakatsuka, H. Kawasaki, S. Yamashita, S. Kohjiya, The Polymerization of β-propiolactone by calcined synthetic hydrotalcite, Bull Chem Soc Jpn, 1979, 52, 2449–2450.
- C. T. Fishel, R. J. Davis, Characterization of magnesium-aluminum mixed oxides by a temperature-programmed reaction of 2-propanol, Langmuir, 1994, 10, 159–165.
DOI: http://dx.doi.org/10.13171/mjc10602007021464sa
Refbacks
- There are currently no refbacks.
Copyright (c) 2020 Mediterranean Journal of Chemistry
