Quantitative Structure-Activity Study against Plasmodium falciparum of a Series of Derivatives of Azetidine-2-Carbonitriles by the Method of Density Functional Theory
Abstract
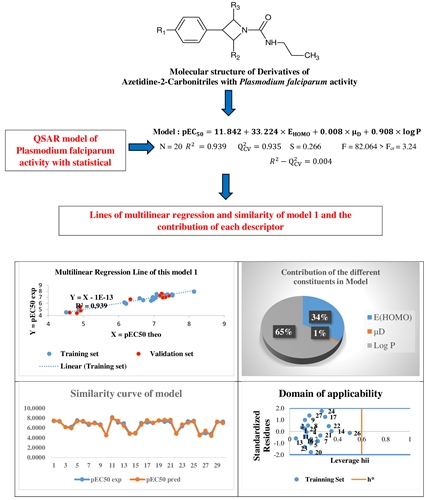
This work deals with a Quantitative Structure-Activity study against Plasmodium falciparum of a series of Azetidine-2-carbonitrile derivatives. Using the MLR and MNLR methods from excel and xlstat software, we have been able to develop two QSAR models based on molecular descriptors and plasmodial activity. Calculation level B3LYP/6-311 G (d, p) was used to determine molecular descriptors. The statistical indicators of the first model obtained by the MLR method are: the regression coefficient found was R2 = 0.939 with a standard deviation S =0.266, Fischer's coefficient F =82.064, and a cross-validation correlation coefficient =0.935. The parameters of the second model developed by the MNLR method are: the regression coefficient R2: de 0.953, a standard deviation S of 0.258, the Fischer's test F of 108.957, and the correlation coefficient of the cross-validation =0.951. Moreover, these models have shown some interesting statistical performance. The energy of the highest occupied molecular orbital (EHOMO), the dipole moment (µD), and the partition coefficient (log P) are the molecular descriptors responsible for the Plasmodium falciparum activity of Azetidine-derivatives 2-carbonitriles. Furthermore, the partition coefficient is the primary descriptor for predicting the biological activity of the studied compounds. From the findings, Eriksson et al. and the external validation criteria of Tropsha used to implement the test are verified and accurate.
Full Text:
PDFReferences
- J. Schantz-Dunn, M. N. NOUR, Malaria, and Pregnancy: a Global Health Perspective, Rev. Obstet. Gynecol., 2009, 2, 186-192.
- World Health Organization, Report of the sixteenth WHOPES working group meeting, Geneva, Switzerland, 2013.
- World Health Organization, World malaria report 2014, Geneva, Switzerland, 2014.
- Organisation Mondiale de la Santé, Plan d’action mondial contre le paludisme (GMAP), 2005.
- M. TANOH, « évaluations des besoins, Draft 1, 2020.
- WHO, Grands lignes du plan d’action de l’OMS pour la lutte contre le paludisme. 1993 - 2000, Amsterdam, Conférence ministérielle sur le paludisme, 1992.
- Y. J. Golvan, Eléments de parasitologie médicale, 4 édition, Paris, Flammarion, 1983.
- M. Gentillini, B. Duflo, Médecine Tropicale, 4 édition, Paris, Flammarion, 1986.
- World Health Organization, World malaria report 2014, Geneva, Switzerland, 2008.
- S. F. Ayad, S. M. Mahmood, A. A. Mohamed, Synthesis and characterization of mono/bis β- lactams by using [2+2] cycloaddition reaction and study antihyperglycemic activity, Best Int. J. Humanit. Med. Sci., 2014, 2, 67-78.
- M. Noolvi, S. Agrawal, H. Patel, A. Badiger,
M. Gaba, A. Zambre, Synthesis, antimicrobial and cytotoxic activity of novel azetidine-2-one derivatives of 1Hbenzimidazole, Arab. J Chem., 2014, 17, 219-226.
- M. T. Chhabria, B. M. Mahajan, P. S. Brahmkshatriya, QSAR Study of a Series of Acyl Coenzyme A (CoA): Cholesterol Acyltransferase Inhibitors Using Genetic Function Approximation, Med. Chem. Res., 2011, 20, 1573-1580.
- V. M. Buha, D. N. Rana, M. T. Chhabria, K. H. Chikhalia, B. M. Mahajan, P. S. Brahmkshatriya, N. K. Shah, Synthesis, Biological Evaluation and QSAR Study of a Series of Substituted Quinazolines as Antimicrobial Agents, Med. Chem. Res., 2013, 22, 4096-4109.
- A. Tropsha, Best Practices for QSAR Model Development, Validation, and Exploitation, Mol. Inform., 2010, 29, 476-488.
- T. I. Oprea, Chemoinformatics in Drug Discovery, WILEY-VCH Verlag, Allemagne, 2005.
- E. A. Rekka, P. N. Kourounakis, Chemistry and Molecular Aspects of Drug Design and Action, CRC Press, 2008.
- C. Hansch, T. Fujita, Additions and Corrections-ρ – σ – π Analysis. A Method for correlation of biological activity and chemical structure, J. Am. Chem. Soc., 1964, 86, 1616-1626.
- S. M. Free, J. W. Wilson, A Mathematical Contribution to Structure-Activity Studies, J Med Chem., 1964, 7, 395-399.
- M. Maetani, N. Kato, V. A. Jabor, F. A. Calil, M. C. Nonato, C. A. Scherer, S. L. Schreiber, Discovery of Antimalarial Azetidine-2-carbonitriles That Inhibit P. falciparum Dihydroorotate Dehydrogenase, ACS Med. Chem. Lett., 2017, 4, 438-442.
- P. J. Taylor, Hydrophobic Properties of Drugs, In Quantitative Drug DesignII, Pergamon Press, Oxford (UK), 1990, 4, 241-294.
- C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Deliv. Rev., 1997, 6, 3-25.
- ChemDraw Ultra, CambridgeSoft©Mulder 875-317589-4732. 1986.
- M. G. Koné, J. S. N’dri, C. G. Kodjo, Combining of DFT and QSAR results to predict the antibacterial activity of a series of azetidinones derived from dapsone as inhibitors of Bacillus Subtilis and Pseudomonas aeruginosa, SDRP J. Comput. Chem. Mol. Model., 2018, 2, 1-8.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, D. J. Fox, Gaussian 09 (Wallingford, CT: Gaussian Inc), 2009.
- Microsoft Excel, Microsoft Office Professionnel. 2016.
- XLSTAT Version 2014.5.03 Copyright Addinsoft 1995-2014 (2014) XLSTAT and Addinsoft are Registered Trademarks of Addinsoft.
- J. S. N’dri, A. L. Kablan, B. Ouattara, M. G. Koné, L. P. Ouattara, C. G. Kodjo, N. Ziao, QSAR studies of the Antifungal activities of α-diaminophosphonates Derived from Dapsone by DFT Method, J. Mater. Phys. Chem., 2019, 7,
-7.
- A. Golbraikh, A. Tropsha, Beware of q2, J Mol Graph Model., 2002, 20, 269-276.
- A. Tropsha, Best Practices for QSAR Model Development, Validation, and Exploitation, Mol. Inform., 2010, 29, 476-488.
- K. Roy, S. Kar, R. N. Das, Statistical Methods in QSAR/QSPR, A Primer on QSAR/QSPR Modeling, Springer cham., 2015, 37-59.
- J. Jaworska, N. N. Jeliazkova, T. Aldenberg, QSAR Applicability Domain Estimation by Projection of the Training Set in Descriptor Space: A Review, Alternatives to laboratory animals,2005, 33, 445-459.
- M. Ghamali, S. Chtita, M. Bouachrine, T. Lakhlifi, Méthodologie générale d’une étude RQSA/RQSP, Rev. Interdiscip., 2016.
- S. Chtita, M. Ghamali, R. Hmamouchi, B. Elidrissi, M. Bourass, M. Larif, T. Lakhlifi, Investigation of Antileishmanial Activities of Acridines Derivatives against Promastigotes and Amastigotes Form of Parasites Using QSAR Analysis, Adv. Phys. Chem., 2016, 1-16.
- T. Asadollahi, S. Dadfarnia, A. Shabani, J. Ghasemi, M. Sarkhosh, QSAR Models for CXCR2 Receptor Antagonists Based on the Genetic Algorithm for Data Preprocessing Prior to Application of the PLS Linear Regression Method and Design of the New Compounds Using In Silico Virtual Screening, Molecules, 2011, 16, 1928-1955.
- S. Chtita, M. Larif, M. Ghamali, M. Bouachrine, T. Lakhlifi, Quantitative structure–activity relationship studies of dibenzo[a,d]cycloalkenimine derivatives for non-competitive antagonists of N-methyl-D-aspartate based on density functional theory with electronic and topological descriptors, J. Taibah Univ. Sci., 2015, 9, 143-154.
- P. P. Roy, K. Roy, On some aspects of variable selection for partial least squares regression models, QSAR Comb. Sci., 2008, 27, 302-313.
DOI: http://dx.doi.org/10.13171/mjc02103241572mgrk
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
