Theoretical Characterization of the Hydrogen Bonding Interaction Sites of Mycolactone C Using the ONIOM Method
Abstract
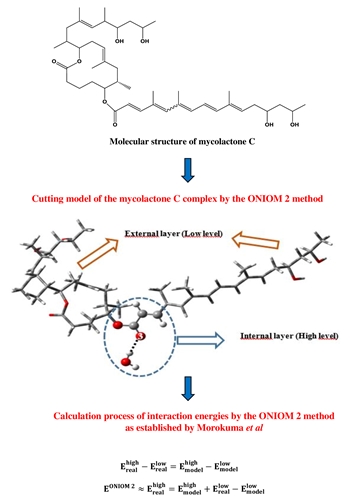
In this work, the ONIOM method, recognized for its effectiveness on large molecules, was used to determine the geometric, energetic, and spectroscopic parameters of hydrogen bond interactions of mycolactone C. Mycolactone C; one of the most virulent forms of toxin, found in Africa and Australia. It has eight (08) oxygen heteroatoms which are all hybridized sp2 and sp3. Using quantum chemistry methods, at the ONIOM level (B3LYP/6-311+G (d, p): AM1), we have determined the preferential binding sites of the hydrogen bonds in the eight mycolactone C oxygen heteroatoms studied. Analysis of the results revealed that the heteroatom O5sp2 is the most suitable site for creating a strong hydrogen bond based on the geometric, energetic (free enthalpy of complexation), and spectroscopic (vibration frequency shifts) parameters. Identifying this O5sp2 heteroatom is a significant step forward in developing a methodology for eradicating the infection and the destructive effects of this toxin.
Full Text:
PDFReferences
- G. E. Anagonou, G. E. Sopoh, C. A. Biaou, Y. T. Barogui, A. C. Wadagni, S. R. Gnimavo, G. A. Ayelo, K. E. Saka, J. G. Houezo, R. C. Johnson, Environmental and behavioural factors associated with Mycobacterium ulcerans infection in the district of Lalo in Benin: A case-control study, Journal of Public Health and Epidemiology, 2021, 13, 54-63.
- H. Simpson, K. Deribe, N. T. Earnest, P. Adebayo, M. Issaka, M. Frimpong, R. Ampadu, R. Phillips, P. Sauderson, J. Carno, Mapping the global distribution of Buruli ulcer : a systematic review with evidence concensus, The Lancet Global Health, 2019, 7, 912-922.
- M. Beissner, K. L. Huber, K. Badziklou, W. A. Halatoko, I. Maman, F. Vogel, E. Piten, K. Helfrich, C. Mengele, J. Nitschke, K. Amekuse, F. X. Wiedemann, A. Dietfenhardt, B. Kobara, K. H. Herbinger, A. B. Kere,
M. Prince-David, G. Bretzel, Loop-Mediated Isothermal Amplification for Laboratory Confirmation of Buruli Ulcer Disease-Towards a Point-of-Care Test, PLoS Negl Trop Dis., 2015, 9, 4219-4230.
- M. Beissner, K. L. Huber, K. Badziklou, W. A. Halatoko, I. Maman, F. Vogel, E. Piten, K. Helfrich, C. Mengele, J. Nitschke, K. Amekuse, F. X. Wiedemann, A. Dietfenhardt, B. Kobara, K. H. Herbinger, A. B. Kere,
M. Prince-David, G. Bretzel, Implementation of a national reference laboratory for Buruli ulcer disease in Togo, PLoS Negl Trop Dis., 2013, 7, 1371-1380.
- M. A. Kabiru, S. Tjip, R. O. van der Werf, F. S. Phillips, J. Sarfo, S. O. Abotsi, W. N. Mireku, K. Asiedu, Y. Stienstra, K. Sandor-Adrian, Short Report: Buruli Ulcer Control in a Highly Endemic District in Ghana: Role of Community-Based Surveillance Volunteers, Am. J. Trop. Med. Hyg., 2015, 92, 115-117.
- B. Forester, G. Demangel, T. Thye, Mycolactone induces cell death by SETD1B dependent degradation of glutathone, PLoS Negl Trop Dis., 2020, 14, 87-99.
- A. Sami, B. Neji, J. Bassem, Derivatives of indoles in anti-tumor activities, Mar. J. Heterocycl Chim., 2020, 19, 124-133.
- H. Marzieh, A. T. Avat, Structural Assessment of Hydrogen Bonds on Methylpentynol-Azide Clusters To Achieve Regiochemical outcome of 1,3-Dipolar cycloaddition Reactions Using Density Functional, ACS Publications, American Chemical Society, 2020, 5, 5964-5975.
- G. R. Desiraju, Designer Crystal: intermolecular interactions, network structures and supramolecular synthons, chemical Communications, 1997, 16, 1475-1482.
- B. Moulton, M. J. Zaworotko, From Molecules to crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids, Chem. Rev., 2001, 101, 1629-1658.
- I. V. Ananyev, N. A. Bokach, V. Y. Kukushkin, Structure-directing sulfur…Metal noncovalent semicoordination bonding, Acta. Cryst., 2020, 76, 436-449.
- A. E. Bela, E. M. J. Pereira, C. T. P. Freire, N. D. Argyriou, J. Eckert, Hydrogen bonds in crystalline D-alanine: diffraction and spectroscopic evidence for difference between enantiomers, Chemistry Crysteng, 2020, 5, 6-12.
- M. G. R. Koné, S. T. Affi, N. Ziao, K. Bamba, E. F. Assanvo, Hydrogen bonding sites in Benzimidazolyl-chalcones molecules: An ab initio and DFT investigation, Journal of Chemical and Pharmaceutical Research, 2015, 7, 805-812.
- S. T. Affi, N. Ziao, K. Bamba, Détermination, par des méthodes ab initio et dft, des sites et énergies de protonation d’une série de molécules d’imidazopyridinyl-chalcones substituées, European Scientific Journal, 2015, 11, 138-148.
- I. Nobeli, S. L. Price, J. P. M. Lommerse, R. Taylor, Hydrogen bonding properties of oxygen and nitrogen acceptors in aromatic heterocycles, J. Comput. Chem., 1997, 18, 2060-2074.
- N. Ziao, C. Laurence, J. Y. Le Questel, Amino nitrogen and carbonyl oxygen in competitive situations, Cryst. Eng. Comm., 2002, 4, 326-335.
- N. Ziao, J. Graton, C. Laurence, J. Y. Le Questel, Amino and Cyano N atoms in competitive situations, Acta Cryst., 2001, 57, 850-858.
- K. Morokuma, ONIOM and Its Applications to Material Chemistry and Catalyses, Bulletin of the Korean Chemical Society, 2003, 24, 797-801.
- L. W. Chung, H. Hirao, X. Li, K. Morokuma, The ONIOM method: its foundation and applications to metalloenzymes and photobiology, Computational Molecular Science, 2012, 2, 327-350.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Jr., Vreven, K. N. Kudin, J. C. Burant, J. M, Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. P. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz., Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. D. J. Martin, Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson,
W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian 03, Revision B.02. Gaussian, Inc., Pittsburgh PA, 2003.
- W. Piao, S. Tsuda, Y. Tanaka, S. Maeda, F. Liu, S. Takahashi, T. Komatsu, Development of azo‐based fluorescent probes to detect different levels of hypoxia, Angewandte Chemie International Edition, 2013, 52, 13028-13032.
- J. Sanchez-Marquez, New advances in conceptual-DFT: an alternative way to calculate the Fukui function and dual descriptor, The Journal of Molecular Modeling, 2019, 25, 19-40.
- S.Tothadi, G. R. Desiraju, Designing ternary cocrystals with hydrogen bonds and halogen bonds, Chemical Communications, 2013, 49, 7791-7793.
- R. Fujiyama, A. Inoule, M. Harada, Transmission Mechanism of Electronic Effects in Cyclophenylene Framework, Journal of Computer Chemistry, 2019, 18, 147-149.
- S. Maeda, Y. Harabuchi, Y. Ono, T. Taketsugu, K. Morokuma, Intrinsic reaction coordinate: calculation, bifurcation, and automated search, International Journal of Quantum Chemistry, 2015, 115, 258-269.
DOI: http://dx.doi.org/10.13171/mjc02104261574mgrk
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
