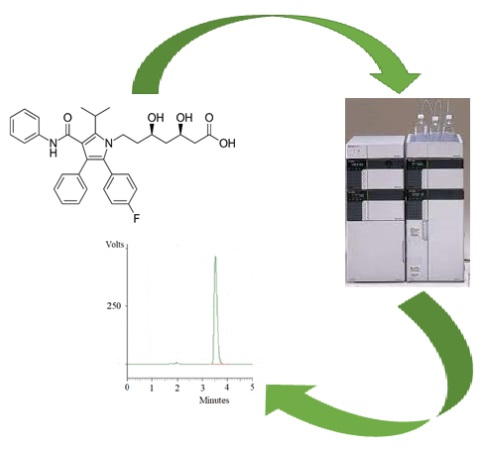
Development and Validation of HPLC Method for the Quantification of Atorvastatin in Pharmaceutical Dosage Forms and Biological Fluid
Abstract
A reverse phase HPLC method was developed for the determination of atorvastatin. The mobile phase involved for the separation was phosphate buffer and acetonitrile with a ratio of 10:1. The HPLC column C18 ODS hypersil column (250 mm×4.6 mm, 5 μm) was used and detected at 215 nm. The run time of the current method was 5 minutes with excellent specificity; no interferences were observed in the pharmaceutical dosage form. The process was validated according to ICH guidelines. The linearity of the proposed method was within the range of 0.25–3.8 µg/ml. The LOD and LOQ values were found to be 0.21 and 0.64 µg/ml. The % recovery and %RSD were within the range of 98–100 %, and ±2% for accuracy, precision, robustness, ruggedness results. All the values are acceptable as per ICH guidelines. As well, this enhanced technique was applied to calculate the amount of atorvastatin in human urine samples. Therefore, the present method is reliable for quantifying atorvastatin in quality control samples in academic and pharmaceutical industries and can easily be used in research development and hospitals.
Full Text:
PDFReferences
- K. J. Udaya, H. M. M. B. Herath, P. V. N. Kaushalya, Method development, validation, and concentration determination of metformin hydrochloride and atorvastatin calcium using UV-visible spectrophotometry, J. Anal. Bioanal. Tech., 2021, 12, 428.
- S. Sun, R. Wang, J. Fan, G. Q. Zhang, H. Zhang, Effects of danshen tablets on pharmacokinetics of atorvastatin calcium in rats and its potential mechanism, Pharma. Bio., 2018, 56, 104–108.
- L. Garza, J. Dols, M. Gillespie, An initiative to improve primary prevention of cardiovascular disease in adults with type II diabetes based on the ACC/AHA(2013) and ADA(2016) guidelines, J. American Associa. Nurse Pract., 2017, 29, 606–611.
- K. Lampropoulos, A. Megalou, G. Bazoukis, G. Tse, A. Manolis, Pre-loading therapy with statins in patients with angina and acute coronary syndromes undergoing PCI, J. Interven. Cardiol., 2017, 30, 507–513.
- O. F. Cruz-Correa, R. B. Leon-Cachon, H. A. Barrera-Saldana, X. Soberon, Prediction of atorvastatin plasmatic concentrations in healthy volunteers using integrated pharmacogenetics sequencing, Pharmacog., 2017, 18, 121–131.
- T. J. Dennison, J. C. Smith, R. K. Badhan, A. R. Mohammed, Fixed-dose combination orally disintegrating tablets to treat cardiovascular disease: formulation, in vitro characterization and physiologically based pharmacokinetic modelling to assess bioavailability, Drug Design, Develop. Ther., 2017, 11, 811–826.
- H. Eng, R. J. Scialis, C. J. Rotter, J. Lin, S. Lazzaro, M. V. Varma, L. Di, B. Feng, M. West M, A. S. Kalgutkar, The antimicrobial agent fusidic acid inhibits organic anion transporting polypeptide–mediated hepatic clearance and may potentiate statin-induced myopathy, Drug Metabol. Dispos., 2016, 44, 692–699.
- P. Virani, R. Sojitra, H. Raj, V. Jain, A review on irbesartan co-administered with atorvastatin for the treatment of cardiac risk, J. Crit. Rev., 2014, 1,25–28.
- M. Manikandan, K. Kannan, S. Thirumurugu, R. Manavalanet, Design and evaluation of amlodipine besilate and atorvastatin calcium tablets, Res. J. Pharm. Bio. Chem. Sci., 2012, 3, 425–434.
- H. Lennernas, Clinical pharmacokinetics of atorvastatin, Clin. Pharma., 2003, 42, 1141–1160.
- M. M. Salim, M. E. E. Sharkasy, M. Walash, F. Belal, Genetic Algorithm with model-updating-based PLS regression for the spectrophotometric determination of clopidogrel, atorvastatin, and aspirin in the presence of its degradation product, J. Appl. Spectros., 2020, 87, 568–578.
- Y. Bilal, K. Selcuk. UV and first derivative spectrophotometric methods for the estimation of atorvastatin in pharmaceutical preparations, J. Adv. Pharm. Res., 2018, 2, 89–94.
- M. A. A. Sobhy, M. A. A. Lobna, A. M. M. Maha, Spectrophotometric determination of atorvastatin calcium and rosuvastatin calcium in bulk and dosage form using p-dimethylaminobenzaldehyde, J. Appl. Pharm., 2017, 9, 233.
- A. Alshabrawy, M. Ahmed, A. Nageh, Sensitive spectrophotometric determination of atorvastatin in pharmaceutical formulation by ion pair complexation with pararosaniline hydrochloride, J. Adv. Pharm. Res., 2017, 1, 193–200.
- A. A. Ramadan, M. Hasna, S. Jenan, Determination of atorvastatin calcium in pure and its pharmaceutical formulations using iodine in acetonitrile by UV-visible spectrophotometric method, Int. J. Pharm. Pharma. Sci., 2015, 7, 427–433.
- M. Sharma, I. Mehta, Surface stabilized atorvastatin nanocrystals with improved bioavailability, safety and antihyperlipidemic potential, Sci. Rep., 2019, 9, 16105.
- S. Dimitra, G. Christos, Kontoyannis, Identification and quantitative determination of atorvastatin calcium polymorph in tablets using FT-Raman spectroscopy, Talanta., 2008, 74, 1066-1070.
- A. Mahesh, Capillary electrophoresis method development for simultaneous determination of atorvastatin and ezetimibe from solid dosage form, J. Young Pharm., 2017, 9, 120–123.
- S. S. Blanka, H. Gabriel, S. S. Istvan, K. Bela, K. Hajnal, Simultaneous determination of atorvastatin and ezetimibe from combined pharmaceutical products by micellar electrokinetic capillary
chromatography, Braz. J. Pharm. Sci., 2017, 53, e16122.
- A. H. Said, S. E. Eman, Y. S. Maissa, A. E. Z. Badr, Development and validation of HPLC and CE methods for simultaneous determination of amlodipine and atorvastatin in the presence of their acidic degradation products in tablets, Acta Pharma., 2016, 66, 479–490.
- W. A. Silva, L. G. de Almeida, F. N. Feiteira, F. S. Semaan, R. Q. Aucelio, R. M. Dornellas, W. F. Pacheco, Novel electrochemical determination of atorvastatin by monitoring the suppression of a lead probe, Anal. Lett., 2021, 54, 541–557.
- F. A. Ali, High sensitivity determination of atorvastatin calcium in pharmaceuticals and biological fluids using adsorptive anodic stripping voltammetry onto surface of ultra-trace graphite electrode, Curr. Anal. Chem., 2018, 14, 92–100.
- A. R. Abdul, A. A. Hussen, M. Mohammad, TLC simultaneous determination of amlodipine, atorvastatin, rosuvastatin and valsartan in pure form and in tablets using phenyl-modified aleppo bentonite, Int. J. Pharm. Pharm. Sci., 2014, 6, 180–188.
- A. G. Nikalje, V. P. Choudhari, Validated TLC method for simultaneous quantitation of atorvastatin, ezetimibe, and fenofibrate in bulk drug and formulations, Acta Chromatogra., 2011, 23, 267–280.
- R. M. Shroff, P. A. Bachhav, S. B. Ganorkar, A. A. Shirkhedkar, S. S. Chalikwar, Analytical and bioanalytical profile for atorvastatin: an exploratory review, Europ. J. Pharma. Med. Res., 2020, 7, 271–282.
- T. B. Deshmukh, S. S. Deo, Development and validation of novel HPTLC method for the simultaneous estimation of atorvastatin calcium and telmisartan in tablet dosage form, Int. J. Pharm. Chem. Bio. Sci., 2018, 8, 82–90.
- K. Wadhwa, A. C. Rana, A review on liquid chromatographic methods for the bioanalysis of atorvastatin, Future J. Pharm. Sci., 2021, 7, 1-19.
- A. N. Waghmare, B. S. Muddukrishna, S. G. Vasantharaju, Analytical method development and validation of simultaneous estimation of amlodipine and atorvastatin by RP-UPLC, Mintage J. Pharm. Med. Sci., 2014, 3, 22–25.
- E. Z. Asma, K. C. Lily, W. Yang, S. Vadim, S. L. C. Diana, Simultaneous LC-MS/MS analysis of simvastatin, atorvastatin, rosuvastatin and their active metabolites for plasma samples of obese patients underwent gastric bypass surgery, J. Pharm. Biomed. Anal., 2019, 164, 258–267.
- D. Hossein, H. Mehrdad, Method validation of amlodipine and atorvastatin by liquid chromatography-mass spectrometry (LC-MS) method in human plasma, Cogent Med., 2016, 3, 1129790.
- M. C. Sakac, Z. Vujic, Z. Vujcic, B. Markovic, D. Vasiljevic, LC-MS/MS method for quantification of atorvastatin, o-hydroxy atorvastatin, p-hydroxy atorvastatin, and atorvastatin lactone in rat plasma, Acta Chromatog., 2016, 28, 281–298.
- B. Xia, Y. Li, Y. Zhang, M. Xue, X. Li, P. Xu, T. Xia, S. Chen, UHPLC-MS/MS method for determination of atorvastatin calcium in human plasma: application to a pharmacokinetic study based on healthy volunteers with specific genotype, J. Pharm. Biomed. Anal., 2018, 160, 428–435.
- C. Liyun, Z. Zhijie, W. Xipei, T. Lan, M. Liping, H. Guodong, L. Heping, Z. Shilong, Simultaneous determination of atorvastatin and its metabolites in human plasma by UPLC-MS/MS, Anal. Met., 2017, 9, 1038–1045.
- A. Zahoor, H. Munir, R. Junaid, S. Hussain, S. Naveed, M. O. Alam, K. Khanum, F. Qamar, S. Khan, A RP-HPLC Method for Simultaneous estimation of Chlorpheniramine Maleate, Paracetamol and Phenylephrine Hydrochloride in Bulk, RADS J. Pharm. Pharm. Sci., 2018, 6, 53–58.
- S. Asif, S. Naveed, K. Usmanghani, M. T. Alam, G. Sarwer, Method development and validation of RP-HPLC method for estimation of Eplerenone in bulk and pharmaceutical formulations, RADS J. Pharm. Pharm. Sci., 2017, 5, 20–26.
- A. Zahoor, H. Munir, S. Hussain, K. Khanum, S. Naveed, K. Usmanghani, Development and Validation of RP-HPLC Method for Quantitative Estimation of Vinpocetine in Intellan Capsule. RADS J. Pharm. Pharm. Sci., 2016, 4, 28–33.
- F. Khalid, S. Gul, M. N. Khan, M. T. Alam, UV spectrophotometric method for estimation of moxifloxacin HCl in tablet dosage form and comparative study of its different brands, RADS J. Pharm. Pharm. Sci., 2015, 3, 41–49.
- S. Shakeel, S. Naveed, K. Usmanghani, G. Sarwar, M. T. Alam. RP-HPLC Simultaneous Analysis of Glimepiride and NSAIDs in Active Pharmaceutical Ingredient, Formulations, and Human Serum, RADS J. Pharm. Pharm. Sci., 2015, 3, 58–64.
- W. Alshitari, F. Al-Shehri, D. A. El-Hady, H. M. Albishri, A simple HPLC method containing greener modifier and slighter temperature elevated for simultaneous determination of three statin drugs in tablets, Acta Chromatogr., 2021, doi:10.1556/1326.2021.00896.
- M. M. Zareh, M. Z. Saad, W. S. Hassan, M. E. Elhennawy, M. K. Soltan, M. M. Sebaiy, Gradient HPLC method for simultaneous determination of eight sartan and statin drugs in their pure and dosage forms, Pharm., 2020, 13, 32.
- K. Wadhwa, A. C. Rana, Development and validation of HPLC-UV based bioanalytical method for the quantification of atorvastatin in rat plasma, World J. Adv. Res. Rev., 2020, 7, 121–132.
- S. Alam, S. Saleem, S. Naveed, H. Dilshad, F. Qamar, T. Alam, H. Sadia, M. Karim, A. Khan, HPLC Method Development and Validation of Atorvastatin Calcium in Bulk and Tablet Dosage Form, RADS J. Pharm. Pharm. Sci., 2018, 6, 83–87.
- F. Hamid, F. Qamar, S. Naveed, S. Saleem, S. Basheer, S. Khan, H. Sadia, K. Usmanghani, A Novel RP-HPLC Method for Simultaneous Determination of Moxifloxacin and Atorvastatin, RADS J. Pharm. Pharm. Sci., 2017, 5, 50–56.
- United States Food and Drug Administration. Guidance for industry: Validation of analytical procedures: methodology Q2B, 1996.
- United States Food and Drug Administration. Guideline for industry: text on validation of analytical procedures: ICH Q2A, 1995.
- International conference on the harmonization of the technical requirements for registration of pharmaceuticals for human use. Validation of analytical procedures: text and methodology ICH Q2(R1), 1996.
- A. A. Judeh, A. Sarief, Y. Umar, O. Ashwaq, S. M. Haque, Development, computational studies and validation of spectrophotometric method of metformin hydrochloride in pharmaceutical formulations, J. Chil. Chem. Soc., 2020, 65, 4895–4899.
DOI: http://dx.doi.org/10.13171/mjc02106301581haque
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
