UV-Vis Spectroscopic Characterization of β-Cyclodextrin-Vanillin Inclusion Complex
Abstract
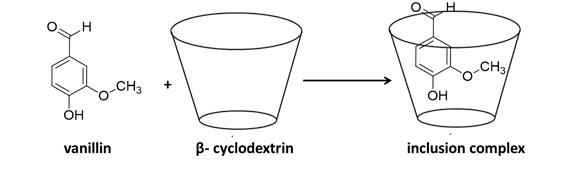
Cyclodextrin molecules can form inclusion complexes with various compounds of appropriate shape and size. The complexation can enhance the solubility and stability of the inclusion guest compound. The stoichiometry and stability constant of the host-guest complex are highly important for physical, chemical, biological, and environmental studies. A simple and rapid spectroscopic method investigated the inclusion of vanillin and β-cyclodextrin (β-CD). The continuous variation technique was used to estimate the stoichiometry of the inclusion complex. The association constant of vanillin with β-CD was determined by using Benesi- Hildebrand and Scott's methods which were calculated to be 179 and 187 M-1, respectively, with the stoichiometry ratio, was 1:1 for the inclusion complex of β-CD with vanillin.
Full Text:
PDFReferences
J. Szejtli, Introduction and General Overview of Cyclodextrin Chemistry, Chem. Rev., 1998, 98, 1743-1754.
M. L. Bender, M. Komiyama, Reactivity and Structure Concepts in Organic Chemistry, Cyclodextrin Chemistry, 1978.
W. Saenger, Cyclodextrin inclusion compounds in research and industry, Angewandte Chemie, 1980, 19, 344-362.
J. Szejtli, Cyclodextrin technology, Kluwer Academic Publishers, 2013.
S. M. Ali, A. Maheshwari, F. Asmat, Complexation of enalapril maleate with β-cyclodextrin: NMR spectroscopic study in solution, Quim. Nova., 2006, 29, 685-688.
A. Roy, S. Saha, M. N. Roy, Study to explore host-guest inclusion complexes of cyclodextrins with biologically active molecules in an aqueous environment, Fluid Phase Equilib., 2016, 425, 252-258.
M. V. Rekharsky, Y. Inoue, Complexation Thermodynamics of Cyclodextrins, Chem. Rev., 1998, 98, 1875-1918.
R. Iacovino, J. V. Caso, F. Rapuano, A. Russo, M. Isidori, M. Lavorgna, G. Malgieri, C. Isernia, Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene:beta-cyclodextrin inclusion complex, Molecules, 2012, 17,
-6070.
K. A. Connors, The stability of cyclodextrin complexes in solution, Chem. Rev., 1997, 97, 1325-1358.
S. He, H. Li, H. Chen, Preparation of light-sensitive polymer/graphene composite via molecular recognition by beta cyclodextrin, J. Mater. Sci., 2018, 53, 14337-14349.
T. Loftsson, M. E. Brewster, Pharmaceutical Applications of Cyclodextrins: Drug Solubilisation and Stabilization, J. Pharm. Sci., 1996, 85, 1017-1025.
Y. Inoue, T. Hakushi, Y. Liu, L. Tong, B. Shen, D. Jin, Thermodynamics of molecular recognition by cyclodextrins. 1. Calorimetric titration of inclusion complexation of naphthalenesulfonates with a-, b-, and c-cyclodextrins: Enthalpy–entropy compensation, Journal of the American Chemical Society, 1993, 115, 475-481.
L. García-Río, R. W. Hall, J. C. Mejuto, P. Rodríguez-Dafonte, The solvolysis of benzoyl halides as a chemical probe determining the polarity of the cavity of dimethyl-b-cyclodextrin, Tetrahedron, 2007, 63, 2208-2214.
L. García-Río, J. C. Mejuto, P. Rodríguez-Dafonte, R. W. Hall, The role of water release from the cyclodextrin cavity in the complexation of benzoyl chlorides by dimethyl-b-cyclodextrin, Tetrahedron, 2010, 66, 2529-2537.
B. G. Poulson, Q. A. Alsulami, A. Sharfalddin, E. F. El Agammy, F. Mouffouk, A. Emwas, L. Jaremko, M. Jaremko, Cyclodextrins: structural, chemical, and physical properties, and applications, Polysaccharides, 2022, 3, 1–31.
M. Prajapati, F. F. Eiriksson, T. Loftsson, Stability characterization, kinetics and mechanism of tacrolimus degradation in cyclodextrin solutions, International Journal of Pharmaceutics, 2020, 586, 119579.
M. Biernacka, T. Ilyich, I. Zavodnik, B. Pałecz, A. Stepniak, Studies of the formation and stability of ezetimibe‐cyclodextrin inclusion complexes, Int. J. Mol. Sci., 2022, 23, 455.
R. L. Carrier, L. A. Miller, I. Ahmed, The utility of cyclodextrins for enhancing oral bioavailability, J. Control. Release, 2007, 123, 78-99.
S. Ray, N. Roy, B. K. Barman, P. Karmakar, P. Bomzan, B. Rajbanshi, V. K. Dakua, A. Dutta, A. Kumar, M. N. Roy, Synthesis and characterization of an inclusion complex of DLaminoglutethimide with β‑cyclodextrin and its innovative application in a biological system: computational and experimental investigations, ACS Omega, 2022, 7, 11208-11216.
M. Cheriet, R. Djemil, A. Khellaf, D. Khatmi, Dopamine family complexes with β-cyclodextrin: molecular docking studies, Polycyclic Aromatic Compounds, 2021, doi: 10.1080/10406638.2021.1970588.
J. Szejtli, Medicinal Applications of Cyclodextrins, Med Res Rev., 1994, 14, 353-386.
X. Zhu, J. Sun, J. Wu, Study on the inclusion interactions of β-cyclodextrin and its derivative with dyes by spectrofluorimetry and its analytical application, Talanta, 2007, 72, 237-242.
E. M. M. Del Valle, Cyclodextrins and their uses: a review, Process Biochem., 2004, 39, 1033-1046.
C. E. de Matos Jensen, R. A. S. dos Santos, A. M. L. Denadai, C. F. F. Santos, A. N. G. Braga, R. D. Sinisterra, Pharmaceutical composition of valsartan: β-Cyclodextrin: Physico-chemical characterization and anti-hypertensive evaluation, Molecules, 2010, 15, 4067-4084.
C. dos Santos, M. P. Buera, M. F. Mazzobre, Phase solubility studies and stability of cholesterol/β-cyclodextrin inclusion complexes, J. Sci. Food Agric., 2011, 91, 2551-2557.
E. Redenti, L. Szente, J. Szejtli, Cyclodextrin complexes of salts of acidic drugs thermodynamic properties, structural features, and pharmaceutical applications, J. Pharm. Sci., 2001, 90, 979-986.
L. Szente, J. Szejtli, Cyclodextrins as food ingredients, Trends Food Sci. Technol., 2004, 15, 137-142.
H. Buschmann, D. Knittel, E. Schollmeyer, New textile applications of cyclodextrins, J. Incl. Phenom. Macrocycl. Chem., 2001, 40, 169-172.
V. A. Marcolino, G. M. Zanin, L. R. Durrant,
M. D. T. Benassi, G. Matioli, Interaction of Curcumin and Bixin with β-Cyclodextrin: Complexation Methods, Stability, and Applications in Food, J. Agric. Food Chem., 2011, 59, 3348-3357.
B. Cheirsilp, J. Rakmai, Inclusion complex formation of cyclodextrin with its guest and their applications, Biol Eng Med., 2016, 2, 1-6.
H. J. Schneider, F. Hacket, V. Rudiger, H. Ikeda, NMR Studies of Cyclodextrins and Cyclodextrin Complexes, Chem. Rev., 1998, 98, 1755-1786.
E. Bertaut, D. Landy, Improving ITC studies of cyclodextrin inclusion compounds by global analysis of conventional and non-conventional experiments, Beilstein J. Org. Chem., 2014, 10, 2630-2641.
T. Pralhad, K. Rajendrakumar, Study of freeze-dried quercetincyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis, Journal of Pharmaceutical and Biomedical Analysis, 2004, 34, 333–339.
R. O. Williams, V. Mahaguna, M. Sriwongjanya, Characterization of an inclusion complex of cholesterol and hydroxypropyl-b-cyclodextrin, European Journal of Pharmaceutics and Biopharmaceutics, 1998, 46, 355–360.
V. T. Karathanos, I. Mourtzinos, K. Yannakopoulou, N. K. Andrikopoulos, Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with b-cyclodextrin, Food Chemistry, 2007, 101, 652–658.
K. Harata, Structural aspects of stereodifferentiation in the solid state, Chemical Reviews, 1998, 98, 1803–1827.
A. Cid-Samamed, J. Rakmai, J. C. Mejuto, J. Simal-Gandara, G. Astray, Cyclodextrins inclusion complex: preparation methods, analytical techniques and food industry applications, Food Chemistry, 2022, 384, 132467.
G. Gonzalez-Gaitano, A. Crespo, G. Tardajos, Thermodynamic investigation (volume and compressibility) of the systems b-cyclodextrin + n-alkyltrimethylammonium bromides + water, Journal of Physical Chemistry B, 2000, 104, 1869–1879.
Y. Saito, K. Watanabe, K. Hashizaki, H. Taguchi, N. Ogawa, T. Sato, H. Ueda, Determination of stability constants for alkanol/a-cyclodextrin inclusion complexes using the surface tension method, Journal of Inclusion Phenomena, 2000, 38, 445–452.
N. Martínez, E. Junquera, E. Aicart, Ultrasonic, density, and potentiometric characterization of the interaction of gentisic and gallic acids with an apolar cavity in aqueous solution. Physical Chemistry Chemical Physics, 1999, 20, 4811–4817.
N. Funasaki, S. Ishikawa, S. Neya, S, Proton NMR study of a-cyclodextrin inclusion of short-chain surfactants, Journal of Physical Chemistry B, 2003, 107, 10094–10099.
M. Kfoury, N. Hadaruga, D. Hadaruga, S. Fourmentin, Cyclodextrins as encapsulation material for flavors and aroma, Encapsulations, 2016, 127-192.
A. Ciobanu, D. Landy, S. Fourmentin, Complexation efficiency of cyclodextrins for volatile flavor compounds, Food Res. Int., 2013, 53, 110–114.
A. Ciobanu, I. Mallard, D. Landy, G. Brabie, D. Nistor, S. Fourmentin, Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers, Food Chem., 2013, 138, 291–297.
M. Kfoury, L. Auezova, S. Fourmentin, H. Greige-Gerges, Investigation of monoterpenes complexation with hydroxypropyl-beta-cyclodextrin, J. Incl. Phenom. Macrocycl. Chem., 2014, 80, 51–60.
M. Kfoury, L. Auezova, H. Greige-Gerges, S. Ruellan, S. Fourmentin, Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies, Food Chem., 2014, 164, 454–461.
C. Moeder, T. O’Brien, R. Thompson, G. Bicker, Determination of stoichiometric coefficients and apparent formation constants for α- and β-CD complexes of terpenes using reversed-phase liquid chromatography, J. Chromatogr. A, 1996, 736, 1-9.
H. Chen, H. Ji, X. Zhou, L. Wang, Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-β-cyclodextrin, Tetrahedron, 2010, 66, 9888-9893.
S. Jiang, J. N. Li, Z. T. Jiang, Inclusion reactions of β-cyclodextrin and its derivatives with cinnamaldehyde in Cinnamomum loureirii essential oil, Eur. Food Res. Technol., 2010, 230, 543-550.
G. Astray, C. Gonzalez-Barreiro, J. C. Mejuto, R. Rial-Otero, J. Simal-Gándara, A review on the use of cyclodextrins in foods, Food Hydrocoll., 2009, 23, 1631-1640.
G. Decock, S. Fourmentin, G. G. Surpateanu, D. Landy, P. Decock, G. Surpateanu, Experimental and theoretical study on the inclusion compounds of aroma components with β-cyclodextrins, Supramol. Chem., 2006, 18, 477-482.
M. Kfoury, D. Landy, S. Ruellan, L. Auezova, H. Greige-gerges, S. Fourmentin, Nootkatone encapsulation by cyclodextrins: Effect on water solubility and photostability, Food Chem., 2017, 236, 41-48.
A. Pîrnău, M. Bogdan, C. G. Floare, NMR Spectroscopic characterization of β-cyclodextrin inclusion complex with vanillin, J. Phys. Conf. Ser., 2009, 182, 012013.
A. Fawzy, I. Zaafarany, K. Khairou, I. Althagafi, J. Alfahemi, Kinetics and Mechanism of Oxidation of Vanillin by Chromium(VI) in Sulfuric Acid Medium, Mod Chem appl., 2016, 4, 179.
B. Bhandari, B. D’Arcy, G. Young, Flavour retention during high-temperature short time extrusion cooking process: a review, International Journal of Food Science and Technology, 2001, 36, 453-461.
C. Jouquand, V. Ducruet, P. Giampaoli, Partition coefficients of aroma compounds in polysaccharide solutions by the phase ratio variation method, Food Chemistry, 2004, 85, 467-474.
J. S. Pagington, Beta-cyclodextrin – the success of molecular inclusion, Chemistry in Britain, 1987, 23, 455-458.
S. Divakar, M. Maheswaran, Structural studies on inclusion compounds of β -cyclodextrin with some substituted phenols, Journal of Inclusion Phenomena and Molecular Recognition in Chemistry, 1997, 27, 113–126.
G. Meryem, K. Rabah, M. Fatiha, N. Leila, B. A. Aziz, D. Imane, M. Rachid, Computational investigation of vanillin@βéta-cyclodextrin inclusion complex: Electronic and intermolecular analysis, Journal of Molecular Liquids, 2021, 321, 114839.
J. Wankar, N. G. Kotla, S. Gera, S. Rasala,
A. Pandit, Y. A. Rochev, Recent Advances in Host-Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering, Adv. Funct. Mater., 2020, 30, 1909049.
P. Job, Studies on the formation of complex minerals in solution and on their stability, Ann. Chimie France, 1928, 9, 113-203.
. S. Renny, L. L. Tomasevich, E. H. Tallmadge, D. B. Collum, Method of continuous variations: applications of Job plots to the study of molecular associations in organometallic chemistry, Angew. Chem. Int. Ed, 2013, 52, 11998–12013.
J-H. Shi, K. Chen, Y. Xu, Characterization of the inclusion interaction between prednisolone and di-O-methyl-β-cyclodextrin: Spectroscopic methods and molecular modeling, Journal of Molecular Liquid, 2014, 194, 172-178.
H. A. Benesi, J. H. Hildebrand, A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons, J Am Chem Soc, 1949, 71, 2703-2707.
. B. Oliveira, Y. S. Meurer, M. G. Oliveira, W. M. Medeiros, F. O. Silva, A. C. Brito, D. L. Pontes, V. F. Andrade-Neto, Comparative study on the antioxidant and anti-toxoplasma activities of vanillin and its resorcinarene derivative, Molecules, 2014, 19, 5898–5912.
X. Wang, Z. Luo, Z. Xiao, Preparation, characterization, and thermal stability of βcyclodextrin/soybean lecithin inclusion complex, Carbohydrate Polymers, 2014, 101, 1027–1032.
S. Saha, A. Roy, K. Roy, M. N. Roy, Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules, Scientific Reports, 2016, 6, 35764.
F. Cramer, W. Saenger, H. Spatz, Inclusion compounds. The formation of inclusion compounds of α-cyclodextrin in aqueous solutions. Thermodynamics and kinetics, J. Am. Chem. Soc., 1967, 89, 14–20.
K. Uekama, T. Fujinaga, T. Hirayama,
M. Otagiri, M. Yamasaki, Inclusion complexations of steroid hormones with cyclodextrins in water and in solid phase, Int J Pharm, 1982, 10, 1-15.
N. Rajagopalan, S. C. Chen, W. S. Chow, A study of the inclusion complex of Amphotericin-B with γ-cyclodextrin, Int J Pharm., 1986, 29, 161-168.
N. A. F. Al-Rawashdeh, K. S. Al-Sadeh, M. B. Al-Bitar, Inclusion Complexes of Sunscreen Agents with β-Cyclodextrin: Spectroscopic and Molecular Modeling Studies, Journal of Spectroscopy, 2013, 2013, 1-11.
K. P. Sambasevam, S. Mohamad, N. M. Sarih, N. A. Ismail, Synthesis and Characterization of the Inclusion Complex of beta-cyclodextrin and Azomethine, Int J Mol Sci, 2013, 14, 3671-3682.
A. Aksamija, A. Polidori, R, Plasson, O. Dangles, V. Tomao, The inclusion complex of rosmarinic acid into beta-cyclodextrin: A thermodynamic and structural analysis by NMR and capillary electrophoresis, Food Chemistry, 2016, 208, 258–263.
M. E. Brewster, T. Loftsson, Cyclodextrins as pharmaceutical solubilizers, Adv Drug Deliv Rev., 2007, 59, 645-666.
DOI: http://dx.doi.org/10.13171/mjc02210111645eteer
Refbacks
- There are currently no refbacks.
Copyright (c) 2022 Mediterranean Journal of Chemistry
