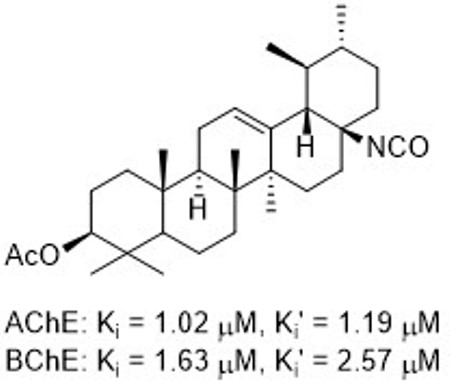
Triterpenoid cholinesterase inhibitors that might improve gait disturbances in Parkinson's disease patients
Abstract
Full Text:
PDFReferences
- P. Anand, B. Singh, A review on cholinesterase inhibitors for Alzheimer's disease, Arch. Pharmacal. Res., 2013, 36, 375-399.
- T.H. Ferreira-Vieira, I.M. Guimaraes, F.R. Silva, F.M. Ribeiro, Alzheimer's disease: Targeting the cholinergic system, Curr. Neuropharmacol., 2016, 14, 101-115.
- J. Grutzendler, J.C. Morris, Cholinesterase inhibitors for Alzheimer's disease, Drugs, 2001, 61, 41-52.
- P. Kasa, Z. Rakonczay, K. Gulya, The cholinergic system in Alzheimer's disease, Prog. Neurobiol. (Oxford), 1997, 52, 511-535.
- A. Lleo, S.M. Greenberg, J.H. Growdon, Current pharmacotherapy for Alzheimer's disease, Annu. Rev. Med., 2006, 57, 513-533.
- V.N. Talesa, Acetylcholinesterase in Alzheimer's disease, Mech. Ageing Dev., 2001, 122, 1961-1969.
- M. Bortolami, D. Rocco, A. Messore, R. Di Santo, R. Costi, V.N. Madia, L. Scipione, F. Pandolfi, Acetylcholinesterase inhibitors for the treatment of Alzheimer's disease - a patent review (2016-present), Expert Opin. Ther. Pat., 2021, 31, 399-420.
- G. Marucci, M. Buccioni, D.d. Ben, C. Lambertucci, R. Volpini, F. Amenta, Efficacy of acetylcholinesterase inhibitors in Alzheimer's disease, Neuropharmacology, 2021, 190, 108352.
- T. Noori, A.R. Dehpour, A. Sureda, E. Sobarzo-Sanchez, S. Shirooie, Role of natural products for the treatment of Alzheimer's disease, Eur. J. Pharmacol., 2021, 898, 173974.
- D.J. Selkoe, Treatments for Alzheimer's disease emerge, Science, 2021, 373, 624-626.
- S. Srivastava, R. Ahmad, S.K. Khare, Alzheimer's disease and its treatment by different approaches: A review, Eur. J. Med. Chem., 2021, 216, 113320.
- S.M. Uddin, A. Al Mamun, T.M. Kabir, G.M. Ashraf, M.N. Bin-Jumah, M.M. Abdel-Daim, Multi-Target Drug Candidates for Multifactorial Alzheimer's Disease: AChE and NMDAR as Molecular Targets, Mol. Neurobiol., 2021, 58, 281-303.
- M.J. Armstrong, M.S. Okun, Diagnosis and Treatment of Parkinson Disease: A Review, JAMA, 2020, 323, 548-560.
- R. Balestrino, A.H.V. Schapira, Parkinson disease, Eur. J. Neurol., 2020, 27, 27-42.
- B.R. Bloem, M.S. Okun, C. Klein, Parkinson's disease, Lancet, 2021, 397, 2284-2303.
- A.B. Malpartida, M. Williamson, D.P. Narendra, R. Wade-Martins, B.J. Ryan, Mitochondrial Dysfunction and Mitophagy in Parkinson's Disease: From Mechanism to Therapy, Trends Biochem. Sci., 2021, 46, 329-343.
- J.H. Chen, T.W. Huang, C.T. Hong, Cholinesterase inhibitors for gait, balance, and fall in Parkinson disease: a meta-analysis, Npj Parkinsons Dis., 2021, 7, 103.
- N. Heise, S. Friedrich, V. Temml, D. Schuster, B. Siewert, R. Csuk, N-methylated diazabicyclo[3.2.2]nonane substituted triterpenoic acids are excellent, hyperbolic and selective inhibitors for butyrylcholinesterase, Eur. J. Med. Chem., 2022, 227, 113947.
- N.V. Heise, D. Ströhl, T. Schmidt, R. Csuk, Stable triterpenoid iminium salts and their activity as inhibitors of butyrylcholinesterase,
J. Mol. Struct., 2022, 1249,131646.
- L. Heller, M. Kahnt, A. Loesche, P. Grabandt, S. Schwarz, W. Brandt, R. Csuk, Amino derivatives of platanic acid act as selective and potent inhibitors of butyrylcholinesterase, Eur. J. Med. Chem., 2017, 126, 652-668.
- L. Heller, S. Schwarz, A. Obernauer, R. Csuk, Allobetulin derived seco-oleananedicarboxylates act as inhibitors of acetylcholinesterase, Bioorg. Med. Chem. Lett., 2015, 25, 2654-2656.
- L. Heller, S. Schwarz, B.A. Weber, R. Csuk, Gypsogenin Derivatives: An Unexpected Class of Inhibitors of Cholinesterases, Arch. Pharm., 2014, 347, 707-716.
- O. Kazakova, I. Smirnova, T. Lopatina, G.N. Giniyatullina, A. Petrova, E. Khusnutdinova, R. Csuk, I. Serbian, A. Loesche, Synthesis and cholinesterase inhibiting potential of A-ring azepano- and 3-amino-3,4-seco-triterpenoids, Bioorg. Chem., 2020, 101, 104001.
- A. Loesche, M. Kahnt, I. Serbian, W. Brandt, R. Csuk, Triterpene-based carboxamides act as good inhibitors of butyrylcholinesterase, Molecules, 2019, 24, 941.
- A. Loesche, A. Koewitsch, S.D. Lucas, Z. Al-Halabi, W. Sippl, A. Al-Harrasi, R. Csuk, Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential, Bioorg. Chem., 2019, 85, 23-32.
- A. Loesche, J. Wiemann, M. Rohmer, W. Brandt, R. Csuk, Novel 12-hydroxydehydroabietylamine derivatives act as potent and selective butyrylcholinesterase inhibitors, Bioorg. Chem., 2019, 90, 103092.
- S. Schwarz, A. Loesche, S.D. Lucas, S. Sommerwerk, I. Serbian, B. Siewert, E. Pianowski, R. Csuk, Converting maslinic acid into an effective inhibitor of acylcholinesterases, Eur. J. Med. Chem., 2015, 103, 438-445.
- S. Schwarz, S.D. Lucas, S. Sommerwerk, R. Csuk, Amino derivatives of glycyrrhetinic acid as potential inhibitors of cholinesterases, Bioorg. Med. Chem., 2014, 22, 3370-3378.
- I.E. Smirnova, O.B. Kazakova, A. Loesche, S. Hoenke, R. Csuk, Evaluation of cholinesterase inhibitory activity and cytotoxicity of synthetic derivatives of di- and triterpene metabolites from Pinus silvestris and Dipterocarpus alatus resins, Med. Chem. Res., 2020, 29, 1478-1485.
- J. Wiemann, A. Loesche, R. Csuk, Novel dehydroabietylamine derivatives as potent inhibitors of acetylcholinesterase, Bioorg. Chem., 2017, 74, 145-157.
- A. Martinez, A. Castro, Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer's disease, Expert Opin. Investig. Drugs, 2006, 15, 1-12.
- G.A. Patani, E.J. LaVoie, Bioisosterism: A Rational Approach in Drug Design, Chem. Rev., 1996, 96, 3147-3176.
- S. Darvesh, R.S. McDonald, A. Penwell, S. Conrad, K.V. Darwesh, D. Mataija, G. Gomez, A. Caines, R. Walsh, E. Martin, Structure-activity relationships for inhibition of human cholinesterases by alkyl amide phenothiazine derivatives, Bioorg. Med. Chem., 2005, 13, 211-222.
- A.K. Gosh, M. Brindisi, Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry, J. Med. Chem., 2019, 63, 2751-2788.
- B. Brandes, S. Hoenke, L. Fischer, R. Csuk, Design, synthesis and cytotoxicity of BODIPY FL labelled triterpenoids, Eur. J. Med. Chem., 2020, 185, 111858.
- S. Braverman, M. Cherkinsky, M.L. Birsa, Carbon dioxide, carbonyl sulfide, carbon disulfide, isocyanates, isothiocyanates, carbodiimides, and their selenium, tellurium, and phosphorus analogues, Sci. Synth., 2005, 18, 65-320.
- S.M. Jain, C.K. Atal, Synthesis of amino derivatives of ursolic acid, Indian J. Chem., Sect. B, 1986, 25B, 427-428.
DOI: http://dx.doi.org/10.13171/mjc02211021650csuk
Refbacks
- There are currently no refbacks.
Copyright (c) 2022 Mediterranean Journal of Chemistry
