Synthesis of highly substituted spiropyrrolidines via 1, 3-dipolar cycloaddition reaction of N-metalated azomethine ylides. A new access to spiropyrroline derivatives
Abstract
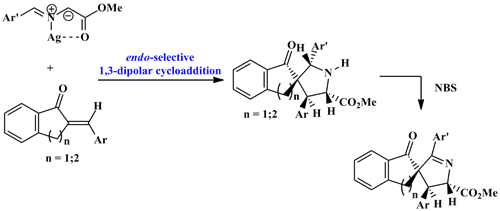
1,3-dipolar cycloaddition of (E)-arylidene-(2H)-indanones 1 (Ar = Ph, p-MeC6H4, p-MeOC6H4, p-ClC6H4) and (E)-2-arylidene-(2H)-tetralones 2 (Ar = Ph, p-MeC6H4, p-MeOC6H4, p-ClC6H4) to N-metalated azomethine ylides 3 generated from methyl N-arylideneglycinate in the presence of silver acetate produces in good yields novel spiro[3,5-(diaryl)-2-carbomethoxypyrrolidine-4:2’-indanones] 4 and spiro[3,5-(diaryl)-2-carbomethoxypyrrolidine-4:2’-tetral-1-ones] 5. The cycloaddition proceeds in regio- and stereoselective manner (100%) at room temperature to afford respectively the syn-endo cycloadducts 4 and 5 via metallo-azomethine ylides. The regio- and stereochemistry of the spiranic adducts has been established on the basis of spectroscopic data and elemental analyses, corroborated by single-crystal X-ray crystallographic analysis of the heterocycles 4cd, 4bb and 5bd. The endo-pyrrolidines 4 were brominated by N-bromosuccinimide to give finally the dehydrobrominated 3,4-dihydro-2H-pyrrole derivatives 6. The spiro-adducts 4 and their corresponding oxidation products 6 are fluorescent in solution.Â
Full Text:
PDFRefbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
