New substituted indene derivatives from bicyclic Baylis-Hillman acetate
Abstract
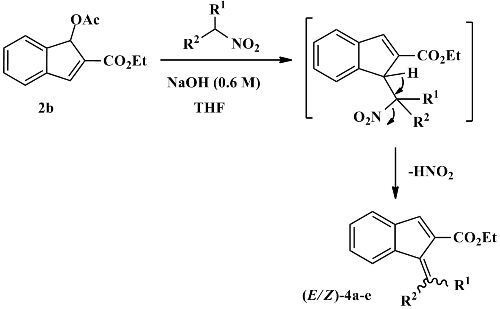
A convenient protocol for the synthesis of highly functionalized indenes 4 has been developed. The coupling reaction of bicyclic Baylis-Hillman acetate 2 with nitroalkane salts in basic conditions led to the corresponding substituted indenes in good yields and high purity.
Full Text:
PDFReferences
G. Majetich, J. M. Shimkus, J. Nat. Prod. 2010, 73, 284-298.
K. Kawazoe, M. Yamamoto, Y. Takaishi, G. Honda, T. Fujita, E. Sezik, E. Yesilada, Phytochemistry 1999, 50, 493-497.
A. N. Nicholson, P. A. Pascoe, C. Turner, C. R. Ganellin, P. M. Greengrass, A. F. Casy, A. D. Mercer, Br. J. Pharmacol. 1991, 104, 270-276.
T. Koike, Y. Hoashi, T. Takai, M. Nakayama, N. Yukuhiro, T. Ishikawa, K. Hirai, O. Uchikawa, J. Med. Chem. 2011, 54, 3436-3444.
R. Kolanos, U. Siripurapu, M. Pullagurla, M. Riaz, V. Setola, B. L. Roth, M. Dukat, R. A. Glennon, Bioorg. Med. Chem. Lett. 2005, 15, 1987-1991.
T. Oda, Y. So, Y. Sato, N. Shimizu, H. Handa, Y. Yasukochi, T. Kasahara, Mutat. Res. 2003, 534, 187-195.
M. R. Schneider, H. Ball, J. Med. Chem. 1986, 29, 75-79.
S. H. Mehdi, R. Hashim, R. M. Ghalib, M. F. C. Guedes da Silva, O. Sulaiman, S. Z. Rahman, V. Murugaiyah, M. M. Marimuthu, J. Mol. Struct. 2011, 1006, 318-323.
A. Korte, J. Legros, C. Bolm, Synlett 2004, 2397-2399.
T. Katoh, T. Akagi, C. Noguchi, T. Kajimoto, M. Node, R. Tanaka, M. Nishizawa, H. Ohtsu, N. Suzuki, K. Saito, Bioorg. Med. Chem. 2007, 15, 2736.
P.-C. Lee, H.-J. Lee, R. Kakadiya, K. Sanjiv, T.-L. Su, T.-C. Lee, Oncogene 2013, 32, 1144-1154.
N. Watanabe, H. Nakagawa, A. Ikeno, H. Minato, C. Kohayakawa, J.-I. Tsuji, Bioorg. Med. Chem. Lett. 2003, 13, 4317-4320.
K. Serge, G. K. Pantelis, P. Daniel, B. Francoise, V. D. V. Patrick, II Farmaco 1999, 54, 678-683.
X. Zhu, H. Tsuji, K. Nakabayashi, S.-I. Ohkoshi, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 16342-16345.
H. G. Alt, A. Köppl, Chem. Rev. 2000, 100, 1205-1222.
C. Li, Y. Zeng, J. Wang, Tetrahedron Lett. 2009, 50, 2956–2959.
H. Kheira, P. Li, J. Xu, J. Mol. Cat. A: Chem. 2014, 391, 168-174.
E. J. Park, S. H. Kim, S. J. Chang, Am. Chem. Soc. 2008, 130, 17268–17269.
G. B. Bajracharya, N. K. Pahadi, I. D. Gridnev, Y. Yamamoto, J. Org. Chem.2006, 71, 6204-6210.
T. Takahashi, Y. Kuzuba, F. Kong, K. Nakajima, Z. Xi, J. Am. Chem. Soc.2005,127, 17188–17189.
K. Bahadur, S. Magar, Y. R. Lee, Org. Lett. 2013, 15, 4288-4291.
E. M. Coyanis, J. L. Panayides, M. A. Fernandes, C. B. Koning, A. L. Willem, J. Organomet. Chem. 2006, 691, 5222-5239.
X. Zhang, W. T. Teo, W. Rao, D.-L. Ma, C.-H. Leung, P. W. H. Chan, Tetrahedron Lett. 2014, 55, 3881–3884.
J. Shao, P. Hu, G. Hong, M. Fang, X. Li, X. Xu, Synlett 2014, 25, 1009–1013.
D. Basavaiah, B. S. Reddy, H. Lingam Tetrahedron 2013, 69, 1994-2003.
D. Eom, S. Park, Y. Park, T. Ryu, P. H. Lee, Org. Lett. 2012, 14, 5392-5395.
H. Kurouchi, H. Sugimoto, Y. Otani, T. Ohwada, J. Am. Chem. Soc. 2010, 132, 807–815.
Z. A. Khan, T. Wirth, Org. Lett. 2009, 11, 229–231.
C. D. Smith, G. Rosocha, L. Mui, R. A. Batey, J. Org. Chem. 2010, 75, 4716–4727.
X. Zhou, H. Zhang, X. Xie, Y. Li, J. Org. Chem. 2008, 73, 3958–3960.
Y. Kuninobu, Y. Nishina, M. Shouho, K. Takai, Angew. Chem. Int. Ed. 2006, 45, 2766-2768.
P. G. Gassman, J. A. Ray, P. G. Wenthold, J. Mickelson, W. J. Org. Chem. 1991, 56, 5143–5146.
Q. Hou, Z. Zhang, F. Kong, S. Wang, H. Wang, Z.-J. Yao, Chem. Commun. 2013, 49, 695-697.
X. Shi, C. Li, Org. Lett. 2013, 15, 1476-1479.
J. Zhao, D. A. Clark, Org. Lett. 2012, 14, 1668-1671.
C. S. Bryan, M. Lautens, Org. Lett. 2010, 12, 2754-2757.
T. Furuta, T. Asakawa, M. Iinuma, S. Fujii, K. Tnaka, T. Kan, Chem. Commun. 2006, 3648–3650.
F. W. Patureau, T. Besset, N. Kuhl, F. Glorius, J. Am. Chem. Soc. 2011, 133, 2154-2156.
S. Taktouk, J. Ben Kraïem, H. Chaabane, J. Lebreton, H. Amri, Med. J. Chem. 2013, 2(5), 658-666.
A. R. Daniewski, J.Kiegiel, J. Org. Chem. 1988, 53, 5535-5535.
K. Samula, B. Cichy, Acta Pol. Pharm.1985, 42, 256; Chem. Abstr. 1986, 105, 171931v.
S. Taktouk, J. Ben Kraïem, H. Amri, Synth. Commun. 2013, 44, 2004-2011.
A. Chamakh, M. M’hirsi, J. Villieras, J. Lebreton, H. Amri, Synthesis 2000, 295–299.
J. Ben Kraïem, H. Amri, J. Soc. Chim.Tunisie 2011, 13, 35-39.
H. Kraïem, T. Turki, H. Amri, Synth. Commun. 2003, 33, 3261-3269.
F. Beji, J. Lebreton, J. Villiéras, H. Amri, Tetrahedron 2001, 57, 9959-9962.
A. Fray, J. Ben Kraïem, H. Amri, Med. J. Chem. 2014, 3(3), 907-915.
I. Yavari, L. Moradi, A. Mokhtarporyani-Sanandej, A. Mirzaei, Helvetica Chimica Acta 2007, 90, 392-394.
DOI: http://dx.doi.org/10.13171/mjc.4.2.2015.12.02.10.25/amri
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
