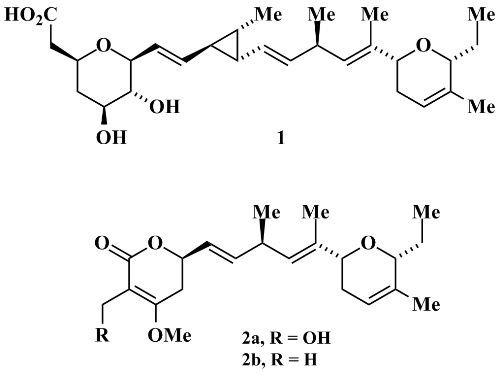
Synthetic Studies of Ambruticin: Preparation of the C1-C8 Tetrahydropyran and the C17-C24 Dihydropyran Segments
Abstract
Full Text:
PDFReferences
- D. T. Connor, R. C. Greenough and M. von Strandtmann, M. J. Org. Chem. 1977, 42, 3664-3669.
– K. Gerth, P. Washausen, G. Höftle, H. Irschik and Reichenbach, J. Antibiotics 1996, 49, 71-75.
– B. Julien, Z. Q. Tian, R. Reid, C. D. Reeves, Chemistry & Biology 2006, 13, 1277-1286.
– a) G. Just, P. Potvin, Can. J. Chem. 1980, 58, 2173-2177;
b) N. J. Barnes, A. H. Davidson, L. R. Hughes, G. Procter and V. Rajcoomar, Tetrahedron Lett. 1981, 22, 1751-1754;
c) Barnes, N. J.; Davidson, A. H.; Hughes, L. R.; Procter, G. J. Chem. Soc., Chem. Comm. 1985, 1292-1294;
d) G. Proctor, A. T. Russell, P. J. Murphy, P. J. Tan and A. N. Mather, A. N. Tetrahedron 1988, 44, 3953-3973;
e) A. H. Davidson, H. Eggleton and I. H. Wallace, J. Chem. Soc., Chem. Commun. 1991, 378-380;
f) I. E. Marko and D. J. Bayston, Tetrahedron 1994, 50, 7141-7156;
g) I. E. Marko and D. J. Bayston, Synthesis 1996, 297-304;
h) H. Wakamatsu, N. Isono and M. Mori, J. Org. Chem. 1997, 62, 8917-8922;
i) P. Varelis and B. L. Johnson, Aust. J. Chem. 1997, 59, 43-51;
j) V. Michelet, K. Adiey, B. Bulic, J. P. Genet, G. Dujardin, S. Rossignol, E. Brown and L. Toupet, Eur. J. Org. Chem. 1999, 2885-2892;
k) J. Yin, I. Llorente, L. A. Villanueva and L. S. Liebeskind, J. Am. Chem. Soc. 2000, 122, 10458-10459;
l) I. Marko, T. Kumamoto and T. Giard, Adv. Synth. Catal. 2002, 1063-1067;
m) V. Michelet, K. Adiey, S. Tanier, G. Dujardin and J. P. Genet, Eur. J. Org. Chem. 2003, 2947-2958.
– a) A. S. Kende, J. S. Mendoza and Y. Fujii, J. Am. Chem. Soc. 1990, 112, 9645-9646;
b) A. S. Kende, J. S. Mendoza and Y. Fujii, Tetrahedron 1993, 49, 8015-8038.
– P. Liu and E. N. Jacobsen, J. Am. Chem. Soc. 2001, 123, 10772-10773.
– a) T. A. Kirkland, J. Colucci, L. S. Geraci, M. A. Marx, K. Schneider, D. E. Kaelin, Jr. and S. F. Martin, J. Am. Chem. Soc. 2001, 123, 12432-12433;
b) S. M. Beberich, R. J. Cherney, J. Colucci, C. Courillon, L. S. Geraci, T. A. Kirkland, M. A. Marx, M. Schneider and S. F. Martin, Tetrahedron 2003, 59, 6819-6832.
– E. Lee, S. J. Choi, H. Kim, H. O. Han, Y. K. Kim, S. J. Min, S. H. Son, S. M. Lim and W. S. Jang, Angew. Chem. Int. Ed. 2002, 41, 176-177.
– S. Hanessian, T. Focken, X. Mi, R. Oza, B. Chen, D. Riston, R. Beaudegnies, J. Org. Chem. 2010, 75, 5601-5618.
– J. Pospisil, I. E. Marko, J. Am. Chem. Soc. 2007, 129, 3516-3517.
– S. Hanessian, T. Focken, R. Oza, Org. Lett. 2010, 12, 3172-3175.
– a) L. Liu, W. Donaldson, Synlett 1996, 103-104;
b) J. M. Lukesh, W. Donaldson, Tetrahedron Lett. 2005, 46, 5529-5531.
– a) M. D. Lewis, J. K. Cha, Y. Kishi, J. Am. Chem. Soc. 1982, 104, 4976-4978;
b) S. J. Danishefsky, J. F. Kerwin, Jr., J. Org. Chem. 1982, 47, 3803-3805;
c) A. Hosomi, Y. Sakata, H. Sakuri, Tetrahedron Lett. 1984, 25, 2383-2386;
d) K. Maruoka, K. Nonoshita, T. Itoh, H. Yamamoto, Chem. Lett. 1987, 2215-2216;
e) for a recent review see: A. M. Gomez, F. Lobo, C. Uriel, J. C. Lopez, Eur. J. Org. Chem. 2013, 7221-7262.
– For a discussion of the effects of pyranyl substitutents on the stereoselectivity of C-glycosylation see: C. G. Lucero, K. A. Woerpel, J. Org. Chem. 2006, 71, 2641-2647.
– a) S. Danishefsky, M. T. Bilodeau, Angew. Chem., Int. Ed. 1996, 35, 1380-1419;
b) S. J. Danishefsky, Aldrichimica Acta 1986, 19, 59-69.
– G. Solladie, E. Arce, C. Bauder, M. C. Carreno, J. Org. Chem. 1998, 63, 2332-2337.
– D. C. Myles, M. H. Bigham, Org. Syn. 1992, 70, 231-239.
– S. J. Danishefsky, W. H. Pearson, D. F. Harvey, C. J. Maring, J. P. Springer, J. Am. Chem. Soc. 1985, 107, 1256-1268.
– a) P. P. Deshpande, K. N. Price, D. C. Baker, J. Org. Chem. 1996, 61, 455-458;
b) Lukesh, W. A. Donaldson, Tetrahedron: Asymmetry, 2003, 14, 757-762;
c) S. Xue, L. He, K. Z. Han, X. Q. Zheng, Q. X. Guo, Carbohydrate Res. 2005, 340, 303-307;
d) P. Deelertpaiboon, V. Reutrakul, S. Jarussophon, P. Tuchinda, C. Kuhakarn, M. Pohmakotr, Tetrahedron Lett. 2009, 50, 6233-6235.
– A. Nowacki, K. Smiataczowa, R. Kasprzykowska, B. Dmochowska, A. Wisniewski, Carbohyd. Res., 2002, 337, 265-272.
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
