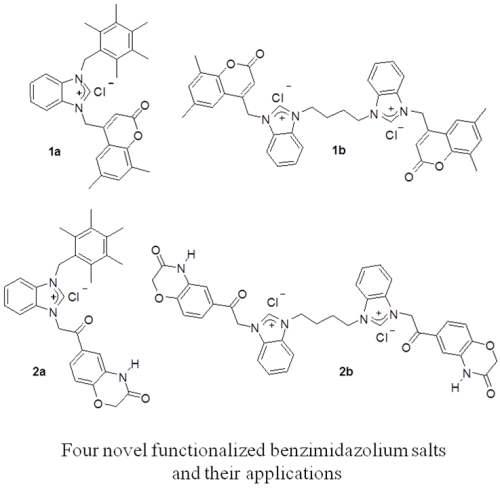
Coumarin or benzoxazinone bearing benzimidazolium and bis(benzimidazolium) salts; involvement in transfer hydrogenation of acetophenone derivatives and hCA inhibition
Abstract
Full Text:
PDFReferences
H.A. Barker, R.D. Smyth, H. Weissbach, J.I. Toohey and B.E. Volcani, Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5,6-dimethylbenzimidazole, J. Biol. Chem., 1960, 235, 480-488.
Keiji Kubo et al., Nonpetide angitensin II receptor antagonists. Synthesis and biological activity of benzimidazoles, J. Med. Chem., 1993, 36, 1772-1784.
K.C.S. Achar, K.M. Hosamani and H.R. Seethaaramareddy, In-vivo analgesic and anti-inflammatıry of newly synthesized benzimidazole derivatives, Eur. J. Med. Chem., 2010, 45, 2048-2054.
K.G. Desai and K.R. Desai, Green route for the heterocyclization of 2-mercaptobenzimidazole into ï¢-lactum segment derivatives containing –CONH- bridge with benzimidazole: Screening in vitro antimicrobial activity with various microorganisms, Bioorg. Med. Chem., 2006, 14, 8271-8279.
Svetlana K. Kotouskaya et al. ,Synthesis and antiviral activity of fluorinated pyrido[1,2-a]benzimidazles, Pharm. Chem. J., 2005, 39, 574-578.
E.R. Cole, G. Crank and Salam-Sheikh, Antioxidant properties of benzimidazoles, J. Agr. Food Chem., 1974, 22, 918.
H.M. Refaat, Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives, Eur. J. Med. Chem., 2010, 45, 2949-2956.
Jeremy M. Travins et al., 1-(3-aryloxaryl)benzimidazole sulfones are liver X receptor agonists, Bioorg. Med. Chem. Lett., 2010, 20, 526-530.
Norbert H. Hauel et al., Structure based design of novel potent nonpeptide thrombin inhibitors, J. Med. Chem., 2002, 45, 1757-1766.
S. Cekirdek, S. Yasar and I. Ozdemir, Palladium (II)-N-heterocyclic carbene complexes: synthesis, characterization and catalytic application, Appl. Organomet. Chem., 2014, 28, 423-431.
S. Demir, M. Yigit and I. Ozdemir, Synthesis of rhodium complexes derived from benzimidazolin-2-yliden ligands and first used for the addition of arylboran to benzonitriles, J. Organomet. Chem., 2013, 732, 21-26.
Dharmendra Kumar et al., Cationic iron(II) complexes of the mixed cyclopentadienyl(cp) and the N-heterocyclic carbine (NHC) ligands as effective precatalysts for the hydrosilyation of carbonyl compounds, J. Organomet. Chem., 2014, 762, 81-87.
H. Turkmen, S. Denizaltı and I. Ozdemir, E. Cetinkaya, B. Cetinkaya, Synthesis and use of mono or bisxylyl linked bis(benzimidazolium) bromides as carbene precursurs for C-C bond formation reactions, J. Organomet. Chem., 2008, 693, 425-434.
N. Gurbuz, S. Demir, I. Ozdemir, B., New 1,2,4,5-tetrakis-(N-imidazoliniummethyl)benzene and 1,2,4,5-tetrakis(N-benzimidazoliniummethyl)benzene salts as N-heterocyclic tetracarbene precursors; synthesis and involvement in ruthenium catalyzed allylation reactions, Cetinkaya and C. Bruneau, Tetrahedron, 2010, 66, 1346-1351.
A. Slamani, S. Demir and I. Ozdemir, Use of benzimidazolium salts for in situ generation of palladium catalysts in Heck reactions in water, Catal. Commun., 2012, 29, 141-144.
R. Noyori and S. Hashiguchi, Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes, Acc. of Chem. Res., 1997, 30, 97-102.
I. Yamada and R. Noyori, Asymmetric transfer hydrogenation of benzaldehydes, Org. Lett., 2000, 2, 3425-3427.
S. Dayan, F. Arslan and N.K. Ozpozan, Ru(II) impregnated Al2O3, Fe3O4, SiO2, and N-coordinate ruthenium (II) arene complexes: Multifunctional catalysts in the hydrogenation of nitroarenes and the transfer hydrogenation of aryl ketones, Appl. Catal. B:Environmental, 2015, 164, 305-315.
G.S. Clark, Coumarin: An aroma chemical profile, Perfumer and Flavonist, 1995, 20, 23-24.
B.G. Lake, Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment, Food and Chem Toxic., 1997, 37, 423-453.
P. Anand, B. Singh and M. Singh, A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease, Bioorg. Med. Chem., 2012, 20, 1175-1180.
D. Yu, M. Suzuki, L. Xie, S.L. Morris-Natschke and K.H. Lee, Recent progress in the development of coumarin derivatives as potent anti-HIV agents, Med. Res. Rev., 2003, 23, 322-345.
Alfonso Maresca et al., Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors, J. Am. Chem. Soc., 2009, 131, 3057-3062.
Alfonso Maresca et al., Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocomarins, J. Med. Chem., 2010, 53, 335-344.
M.G. Nair, D.C. Salter, R.L. Kisliuk, Y. Gaumont and G. North, Folate analogs.22.Synthesis and biological evaluation of two analogs of dihydro folic acid possesing a 7,8-dihydro-8-oxapterin ring system, J. Med. Chem., 1983, 26, 1164-1168.
Y. Katsura, S. Nishino and H. Takasugi, Studies on antiulcer drugs. I.Synthesis and antiulcer activities of imidazo[1,2-alpha]pyrdnyl-2-oxobenzoxazolidines-3-oxo-2H-1,4-benzoxazines and related compounds, Chem. Pharm. Bull., 1991, 39, 2937-2943.
M. Kajino, Y. Shibouta, K. Nishikawa and K. Megura, Synthesis and biological activities of new 2-substitute 1,4-benzoxazine derivatives, Chem. Pharm. Bull., 1991, 38, 2896-2905.
S. Wahidulla and J.J. Bhattaeharje, Benzoxazinoids from Acanthus illicifolius, J. Indian Inst. Sci., 2001, 81, 485-490.
Liang Fang et al., Synthesis of benzo[b][1,4] oxazin-3(4H)-ons via smiles rearrengement for antimicrobial activity, Med. Chem. Res., 2011, 20, 670-677.
Xiaokai Li et al., Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: Novel antibacterial agent against Mycobacterim tuberculosis, Bioorg. Med. Chem. Lett., 2010, 20, 6306-6309.
R. Fringuelli, N. Giacche, L. Milanese, E. Cenci and F. Macchiarulo, Bulky 1,4-benzoxazine derivatives with antifungal activity, Bioorg. Med. Chem., 2009, 17, 3838-3846.
C.F. Turk, J. Krapcho, I.M. Miche and I. Weinryb, Synthesis and central nervous system activity of 2-aryliden-4-aminoalkyl-2H-1,4-benzoxazen-3(4H)-ones and related compounds, J. Med. Chem., 1997, 20, 730-732.
A. Thiry, J.M. Dogne, C.T. Supuran and B. Masareel, Carbonic anhydrase inhibitors of anticonvulsant agents, Curr. Top Med. Chem., 2007, 7, 855-864.
C.T. Supuran, Carbonic anhydrases: Novel therapeutic application for inhibitors and activators, Nat. Rev. Drug Discov., 2008, 7, 168-181.
A. Thiry, J.M. Dagne, B. Masareel and C.T. Supuran, Targeting tumor-associated carbonic anhydrase IX in cancer therapy, Trends Pharmacol. Sci., 2006, 27, 566-573.
James M. McKiernan et al., Expression of the tumor-associated gene MN: A potential biomarker for human renal cell carcinoma, Cancer. Res., 1997, 57, 2362-2365.
A. Scozzafava, A. Mastrolorenzo and C.T. Supuran, Carbonic anhydrase inhibitors and activator and their use in therapy, Expert Opin. Ther. Pat., 2006, 16, 1627-1664.
C.T. Supuran, A. Scozzafava and A. Casini, Carbonic anhydrase inhibitors, Med. Res. Rev., 2003, 23, 146-189.
K.S. Smith and J.G. Ferry, Prokaryotic carbonic anhydrases, FEMS Microbial Rev., 2000, 24, 335-366.
T. Stams and D.W. Christianson, X-ray crystallographic studies of mammalian carbonic anhydrase isozymes. In: Chegwidden WR, Carter ND, Edwards YH, eds. X-ray crystallographic studies of mammalian carbonic anhydrase isozymes. The Carbonic Anhydrases: New Horizons. Boston: Birkhauser Verlag, 2000, 159-174.
S. Pastorekova, S. Parkilla, J. Pastorek and C.T. Supuran, Carbonic anhydrases: Current state of art, therapeutic applications and future prospects, J. Enzyme Inhib. Med. Chem., 2004, 19, 199-229.
Isao Nishimori et al., Carbonic anhydrase inhibitors: Claning, characterization and inhibiton studies of the cytosolic isoenzyme III with sulphonamides, Bioorg. Med. Chem., 2007, 15, 7229-7236.
D. Vullo, M. Franchi, E. Gallori, A. Scozzafava and C.T. Supuran, Carbonic anhydrase inhibitors.Inhibiton of mitochondrial isoenzyme V with aromatic and heterocyclic sulphonamides, J. Med. Chem., 2004, 47, 1272-1279.
Isao Nishimori et al., Carbonic anhydrase inhibitors. The mithocondrial isoenzyme VB as a new target for sulfonamide and sulfomate inhibitors, J. Med. Chem., 2005, 48, 7860-7866.
Isao Nishimori et al., Carbonic anhydrase inhibitors. DNA claning, characterization and inhibiton studies of human secretary isoform VI, a new targer for sulfonamide and sulfomate inhibitors, J. Med. Chem., 2007, 50, 381-388.
Daniela Vullo et al., Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isoenzyme VII with aromatic and heterocyclic sulphonamides, Bioorg. Med. Chem. Lett., 2005, 15, 971-976.
Daniela Vullo et al., Carbonic Anhydrase inhibitors. Inhibiton of transmembrane isoenzyme XII with sulfonamides- a new target for design of antitumor and antigloucoma drugs?, Bioorg. Med. Chem. Lett., 2005, 15, 963-969.
M.S. Frasinyuk, V.I. Vinogradova, S.P. Bondarenko and V.P. Khilya, Synthesis of cytosine derivatives of coumarins, Chem. Nat. Comp., 2007, 43, 176-180.
I. Ozdemir, N. Sahin, S. Demir and B. Cetinkaya, In situ generated 1-alkylbenzimidazoles-palladium catalyst for the Suzuki coupling of aryl chlorides, J. Molec. Catal. A, 2005, 234, 181-185.
O. Arslan, B. Nalbantoglu, N. Demir, H. Ozdemir and O.I. Kufrevioglu, A new method for the purification of carbonic anhydrase izoenzymes by affinity chromatography, Turk. J. Med. Sci., 1996, 26, 163-166.
T.H. Maren, A simplified micromethod for the determination of carbonic anhydrase and its inhibitors J. Pharm. Exp. Ther., 1960, 130, 2629-2634.
DOI: http://dx.doi.org/10.13171/mjc.4.5.2015.17.10.08.51/karatas
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
