Microwave-assisted synthesis of new aryliminothiazolylidene- 2-thiazolidin-4-ones and their azarhodacyanines analogues
Abstract
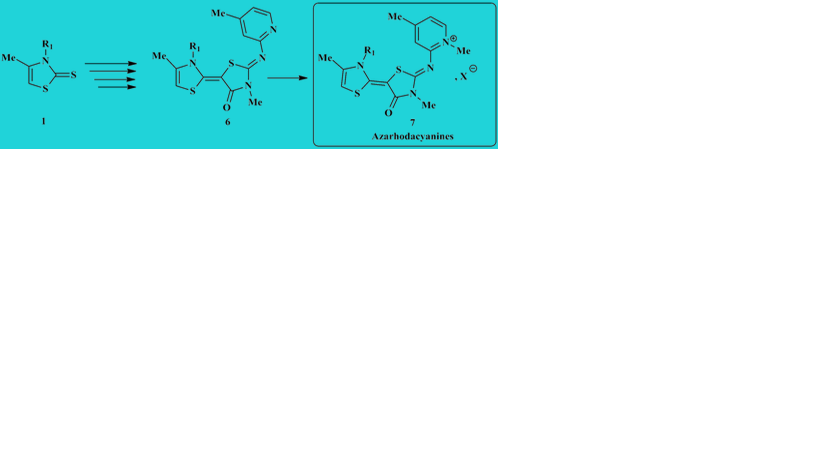
 We here report an efficient microwave-assisted protocol for the synthesis of new arylimino-thiazolylidene-2-thiazolidin-4-ones 6 and their azarhodacyanines derivatives 7 with quantitative yield from 2'-(methylthio)-4'-oxo-3H,4'H-[2,5-bithiazolylidene]-3'-ium tosylates 5 and 2-arylimino-5-(thiazol-2(3H)-ylidene) thiazolidin-4-ones 6, respectively, using as starting material the 4-thiazoline-2-thiones 1 and 3-methyl-2-thioxo-1,3-thiazolidin-4-one 3. The transformation of the tosylate salts 5 into their arylimino derivatives 6 has not been reported to date.
Full Text:
PDFReferences
- K.Tsuji, H. Ishikawa, Bioorg. Med. Chem. Lett., 1994, 4, 1601-1606.
- B. Lopez-Garcia, A. Veyrat, E. Perez-Paya, L. Gonzalez-Candelas, J.F. Marcos, Int. J. Food Microbiol., 2003, 89, 163-170.
- R. Mgonzo, A. Geronikaki, P.N. Kourounakis, Pharmazie, 1995, 50, 505–506.
- S.B. Gomha, K.D. Khalil, Molecules, 2012, 17, 9335-9347.
- M.L. Barreca, A. Chimirri, L. De Luca, A.M. Monforte, P. Monforte, A. Rao, M. Zappalà , J. Balzarini, E. De Clercq, C. Pannecouque, M. Witvrouw, Bioorg. Med. Chem. Lett., 2001, 11, 1793-1796.
- G.Aridoss, S. Amirthaganesan, M.S. Kim, J.T. Kim, Y.T. Jeong, Eur. J. Med. Chem., 2009, 44, 4199-4210.
- R. Ottana, R. Maccari, M. L. Barreca, G. Bruno, A. Rotondo, , A. Rossi, C. Giuseppa, R. Di Paola, L. Sautebin, S. Cuzzocrea, M.G. Vigorita, Bioorg. Med. Chem., 2005, 13, 4243-4252.
- R.V.P. Mujeebur, S. Mukhtar, W.H. Ansari, G. Lemiere, Eur. J. Med. Chem., 2005, 40, 173-184.
- P. Vicini, A. Geronikaki, M. Incerti, F. Zani, J. Deardan, M. Hewitt, Bioorg. Med. Chem., 2008, 16, 3714-3724.
- B. Goel, T. Ram, R. Tyagi, E. Bansal, A. Kumar, D. Mukherjee, Sinha, J. N. Eur. J. Med.Chem.,1999, 31, 265-269.
- M. G. Vigorita, R. Ottana, F. Montforte, R. Maccari, A. Trovato, M. Montforte, M. F. Taviano, Bioorg. Med. Chem. Lett., 2001, 11, 2791-2794.
- M. G. Vigorita, R. Ottana, F. Montforte, R. Maccari, A. Trovato, M. Montforte, M. F. Taviano, Bioorg. Med. Chem. Lett., 2001, 11, 2791-2794.
- H. Chen, L. Jiao, Z. Guo, X.L. Li, C. Ba, J. Zhang, Carbohydr. Res., 2008, 343, 3015-3020.
- N. Cesur, Z. Cesur, N. Ergenç, M. Uzun, M. Kiraz, O. Kasimoglu, D. Kaya, Arch. Pharm., 1994, 327, 271-272.
- K. Omar, A. Geronikaki, P. Zoumpoulakis, C. Camoutsis, M. Soković, Ćirić, A. GlamoÄlija, J. Bioorg. Med. Chem., 2010, 18, 426-432.
- P. Vicini, A. Geronkiaki, M. Incerti, F. Zani, J. Dearden, Hewitt, M. Bioorg. Med. Chem., 2008, 16, 3714-3724.
- P. Vicini, A. Geronikaki, K. Anastasia, M. Incerti, F. Zani, Bioorg. Med. Chem., 2006, 14, 3859-3864.
- L.B. Chen, Ann. Rev. Cell. Biol., 1988, 4, 155-181.
- M. Kawakami, K. Koya, T. Ukai, N. Tatsuta, A. Ikegawa, K. Ogawa, T. Shishido, L.B. Chen, J. Med. Chem., 1997, 40, 3151-3160.
- a) K. Takasu, H. Inoue, H. S. Kim, M. Suzuki, T. Shishido, Y. Wataya, M. Ihara, J. Med. Chem., 2002, 45, 995-998; b) K. Pudhom, K. Kasai, H. Terauchi, H. Inoue, M. Kaiser, R. Brun, M. Ihara, K. Takasu, J. Bioorg. Med. Chem., 2006, 14, 8550-8563.
- K. Takasu, K. Pudhom, M. Kaiser, R. Brun, M. Ihara, J. Med. Chem., 2006, 49, 4795-4798.
- S. Kasmi-Mir, A. Djafri, J. Hamelin, L. Paquin., J.P. Bazureau, M. Rahmouni, Synth. Commun., 2007, 37, 4017-4034.
- W.J. Humphlett, R. Lamon, J. Org. Chem., 1964, 29, 2146-2148.
- A. Hantzsch, J.H. Weber, Ber. Dtsch. Chem. Ges., 1887, 20, 3118-3132.
- W.J. Humphlett, R.W. Lamon, J. Org. Chem., 1964, 29, 2148-2150.
- C. Roussel, R. Gallo, M. Chanon, J. Metzger, J. Chem. Soc. Perkin Trans II, 1974, 1304-1306.
- L.W. Jones, K.S. Narayan, C.E. Shapiro, T.W. Sweatman, J. Chemother., 2005, 17, 435-440.
- F.G. Moers, J.J. Steggerda, J. Inorg. Nucl. Chem., 1968, 30, 3217-3222.
DOI: http://dx.doi.org/10.13171/mjc.2.6.2014.16.03.23
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
