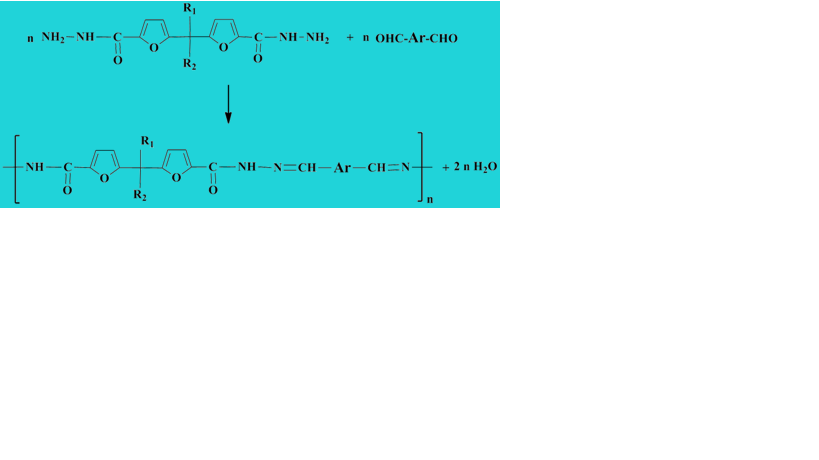
New polyacylhydrazone dynamers incorporating furan moieties
Abstract
Full Text:
PDFReferences
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart. Angew Chem Int Edit, 2002, 41, 898-952.
- J. M. Lehn, Proc. Natl. Acad. Sci. USA, 2002, 99, 4763-4768.
- J.M. Lehn, Science, 2002, 295, 2400-2403.
- J .M. Lehn, Prog.Polym. Sci., 2005, 30, 814-831.
- N. Sreenivasachary, D. T. Hickman, D. Sarazin, J. M. Lehn, Chem. Eur. J., 2006, 12, 8581-8588.
- J. M. Lehn, in Supramolecular Science: Where It Is and Where It Is Going,eds. Ungaro, R. & Dalcanale, E. (Kluwer, Dordrecht, The Netherlands), 1999, pp. 287-304.
- J.M. Lehn, Chem. Eur. J., 1999, 5, 2455–2463.
- G. R. L. Cousins, S.-A. Poulsen, J. K. M. Sanders, Curr. Opin. Chem. Biol., 2000, 4, 270-279
- O. Ramström, J.M. Lehn, Nat. Rev. Drug Discovery, 2001, 1, 26-36.
- T. Ono, T. Nobori, J.M. Lehn, Chem. Commun., 2005, 1522-1524.
- T. Nishinaga, A. Tanatani, K. Oh, J. S. Moore, J. Am. Chem. Soc., 2002, 124, 5934-5935.
- R. Nguyen, I. Huc, Chem. Commun., 2003, 942-943.
- L. M. Hayden, W. K. Kim, A. P. Chafin, G. A. Lindsay, Macromolecules, 2001, 34, 1493-1495.
- J. L. Schmitt, J. M. Lehn, Helv. Chim. Acta., 2003, 86, 3417-3426.
– B. Gyarmati, Ã. Némethy, A. Szilágyi, European Polymer Journal, 2013, 49, 1268–1286.
- W. G. Skene, J. M. Lehn, Proc. Natl. Acad. Sci USA, 2004, 101, 8270-8275.
- G. A. Roberts, I. M. Thomas, Makromol Chem, 1981, 182, 2611-2618.
- W. D. Emmons,US Patent 4, 1980, 210-565; Chem. Abstr., 1980, 60,75763.
- H. D. De Witt, US Patent 3, 1964, 124-559.
- T. Ono, S. Fujii, T. Nobori, J. M. Lehn, Chem. Commun., 2007, 4360-4362.
- T. Maeda, H. Otsuka, A. Takahara, Progress in Polymer Science, 2009, 34, 581-604.
- J. Yu, H. Deng, F. Xie, W. Chen, B. Zhu, Q. Xu, Biomaterials, 2014, 35, 3132-3144.
- M. N. Belgacem, A. Gandini, Monomers, 2008, 39-66
- S. Gharbi, A. Gandini, Acta Polym., 1999, 50, 293-297.
- S.Gharbi, A. Afli, R. El Gharbi, A. Gandini, Polym.Int., 2001, 50, 1-6.
- S. Abid, R. El Gharbi, A. Gandini, Polymer, 2004, 45, 5793-5801
- Afli, A., S. Gharbi, R. El Gharbi, Y. Le Bigot, A. Gandini, Eur. Polym. J., 2002, 38, 667-673.
- A. Afli, S. Gharbi, R. El Gharbi, e–Polymers, 2008, 64, 1-8.
- S. Abid, R. El Gharbi, A. Gandini, Polymer, 2004, 45, 6469-6478.
- S.Abid, S. Matoussi, R. El Gharbi, A. Gandini, Polym. Bull., 2006, 57, 43-50.
- L. Ben Maktouf, I. Ghorbel, A. Afli, S. Abid, A. Gandini, Polymer Bulletin, 2011, 67, 1111-1122.
DOI: http://dx.doi.org/10.13171/mjc.2.6.2014.08.03.23
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
