Insight into the interaction between α-lapachone and bovine serum albumin employing a spectroscopic and computational approach
Abstract
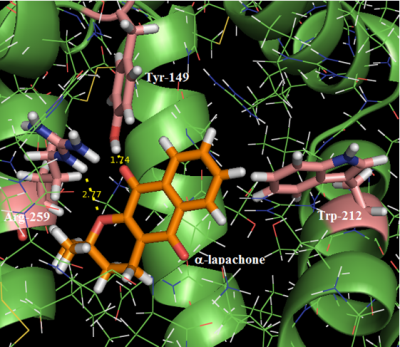
Serum albumin is the most abundant protein in blood plasma; among its functions is the transport of a high variety of drugs in the body. Quinones show several biological and pharmacological activities, such as anti-malarial, antitumor, anti-microbial, anti-inflammatory and anti-parasitic. We report fluorescence and circular dichroism (CD) spectroscopic studies to try to understand the interaction process between α-lapachone (α-LAP) and bovine serum albumin (BSA). Studies using computational methods, such as molecular docking, were performed to identify the main cavity in which this interaction occurs as well as the type of intermolecular interactions between the amino acid residues from albumin and the ligand. The BSA fluorescence quenching by added α-LAP is a static process, indicating an initial association BSA: α-LAP. The Ka and Kb values for the interaction BSA: α-LAP are in the range 105-104 L∙mol-1, indicating a strong binding between these two species. CD data show that there is no significant perturbation on the secondary structure of the protein with binding. The negative ΔGo values are consistent with spontaneous binding occurring endothermically (ΔHo = 127 kJ∙mol-1), and possibly driven by hydrophobic factors (ΔSo = 0.526 kJ∙mol-1∙s-1). The number of binding sites (n) indicates the existence of just one main binding site in BSA for α-LAP, with molecular docking results showing that it binds preferentially to the albumin in the domain IIA, where the Trp-212 residue is located. The ligand interacts via hydrogen bond with Arg-259 and Tyr-149 residues and via T-stacking with the fluorophore Trp-212 residue.
Full Text:
PDFReferences
T.L.G. Lemos, F.J.Q. Monte, A.K.L. Santos, A.M. Fonseca, H.S. Santos, M.F. Oliveira, S.M.O. Costa, O.D.L. Pessoa, R. Braz-Filho, Nat. Prod. Res. 2007, 21, 529-550.
L. F. Fieser, E. Berliner, F. Bondhus, F. C. Chang, W. G. Dauben, M. G. Ettlinger, G. Fawaz, M. Fields, M. Fieser, C. Heidelberger, H. Heymann, A. M. Seligman, W. R. Vaughan, A. G. Wilson, E. Wilson, M.I. Wu, M. T. Leffler, K. E. Hamlin, R. J. Hathaway, E. J. Matson, E. E. Moore, M. B. Moore, R. T. Rapala, H. E. Zaugg, J. Am. Chem. Soc. 1948, 70, 3151-3162.
A.R. Burnett, R.H. Thomson, J. Chem. Soc. C, 1967, 2100-2104.
H. Hussain, K. Krohn, V.U. Ahmad, G.A. Miana, I.R. Green, ARKIVOC, 2007, II, 145-171.
E. Pérez-Sacau, R.G. Diaz-Peñate, A. Estévez-Braun, A.G. Ravelo, J.M. GarcÃa-Castellano, L. Pardo, M. Campillo, J. Med. Chem. 2007, 50, 696-706.
A. S. Cunha, E. L. S. Lima, A. C. Pinto, A. Esteves-Souza,. A. Echevarria, C. A. Camara, M. D. Vargas, J. C. Torres, J. Braz. Chem. Soc. 2006, 17, 439-442.
E. N. Silva Júnior, M. C. B. V. de Souza, A. V. Pinto, M. C. F. R. Pinto, M. O. F. Goulart, F. W. A. Barros, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. de Moraes, V. F. Ferreira, Bioorg. Med. Chem. 2007, 15, 7035-7041.
Kim, S. O.; Kwon, J. I.; Jeong, Y. K.; Kim, G. Y.; Kim, N. D.; Choi, Y. H.; Biosci. Biotechnol. Biochem. 2007, 71, 2169-2174.
K. A. Majorek, P. J. Porebski, A. Dayal, M. D. Zimmerman, K. Jablonska, A. J. Stewart, M. Chruszcz, W. Minor, Mol. Immunol. 2012, 52, 174–182.
K. Taguchi, V.T.G. Chuang, T. Maruyama, M. Otagiri, J. Pharm. Sci. 2012, 101, 3033-3046.
B. K. Paul, A. Samanta, N. Guchhait, J. Phys. Chem. B, 2010, 114, 6183–6196.
D. C. Carter, X. M. He, S. H. Munson, P. D. Twigg, K. M. Gernert, M. B. Broom, T. Y. Miller, Science, 1989, 244, 1195-1198.
S. Sugio, S. Kashima, S. Mochizuki, M. Noda, K. Kobayashi, Protein Eng. Des. Sel. 1999, 12, 439-446.
A. Bujacz, Acta Cryst. 2012, D68, 1278-1289.
J.R. Lakowicz. Principles of Fluorescence Spectroscopy, 1st ed.; Springer New York, U.S.A., 2006; pp. 923–928.
J. Liu, J.N. Tian, J. Zhang, Z. Hu, X. Chen, Anal Bioanal Chem. 2003, 376, 864-867.
J. Tian, X. Liu, Y. Zhao, S. Zhao, J. Luminesc. 2007, 22, 446-454.
M.R. Eftink, C.A. Ghiron, Anal Bioanal Chem. 1981, 114, 199-227.
D. Brune, S. Kim, Biophysics, 1993, 90, 3835-3839.
M.R. Eftink. Fluorescence Quenching Reactions: Probing Biological Macromolecular Structures. In: Biophysical Biochemical Aspects of Fluorescence Spectroscopy, 1st ed.; T.G. Dgurvy,; Plenum Press, New York, U.S.A., 1991, Vol. 1, pp. 1-41.
A. Satheshkumar, K.P. Elango, Spectrochim. Acta Mol. Biomol. 2014, 130, 337-343.
O. A. Chaves, A. P. O. Amorim, L. H. E. Castro, C. M. R. Sant’Anna, M. C. C. de Oliveira, D. Cesarin-Sobrinho, J. C. Netto-Ferreira, A. B. B. Ferreira, Molecules. 2015, 20, 19526-19539.
I.E. Borissevitch, T.T. Tominaga, H. Imasato, M. Tabak, J. Luminesc. 1996, 69, 65–76.
W. He, Y. Li, J. Tian, H. Liu, Z. Hu, X. Chen, J. Photochem. Photobiol. A: Chem. 2005, 174, 53-61.
P. D. Ross, S. Subramanian, Biochemistry 1981, 20, 3096-3102.
Z. Cheng, R. Liu, X. Jiang, Spectrochim. Acta Mol. Biomol. 2013, 115, 92-105.
J. Li, J. Li, Y. Jiao, C. Dong, Spectrochim. Acta Mol. Biomol. 2014, 118, 48-54.
S. Y. Venyaminov, J. T. Yang. In Determination of protein secondary structure. Circular dichroism and the conformational analysis of biomolecules, ed. by G. D. Fasman, Plenum Press, New York, EUA, 1996, pp. 69-80.
P. Yang, F. Gao. The principle of bioinorganic chemistry, Science Press, Beijing, 2002, pp. 349-360.
W.Y. He, Y. Li, H. Z. Si, Y. M. Dong, F. L. Sheng, X. J. Yao, Z. D. Hu, J. Photochem. Photobiol. A: Chem. 2006, 182, 158-165.
A. Varlan, N. Hillebrand, Mol. 2010, 15, 3905-3919.
Y. Yue, Y. Zhang, J. Qin, X. Chen, J. Mol. Struc. 2008, 888, 25-30.
Y. Yue, Y. Zhang, Y. Li, J. Zhu, J. Qin, X. Chen, J. Luminesc. 2008, 128, 513-516.
B.K. Paul, A. Samanta, N. Guchhait, J. Phys. Chem. B, 2010, 114, 6183–6196.
M. Fasano, S. Curry, E. Terreno, M. Galliano, G. Fanali, P. Narciso, S. Notari, P. Ascenzi, IUBMB Life 2005, 57, 787-796.
M.J.S. Dewar, E.G. Zoebisch, E.F. Healy, J.J.P. Stewart, J. Am. Chem. Soc. 1985, 107, 3902-3909.
C. A. C. Ferreira, V. F. Ferreira, A. V. Pinto, R. S. C. Lopes, M. C. R. Pinto, A. J. R. Silva, An. Acad. Bras. Ci. 1987, 59, 5-8.
G. Jones, P. Willett, R.C. Glen, A.R. Leach, R. Taylor, Journal of Molecular Biology 1997, 267, 727-748.
O. Korb, T. Stützle, T.E. Exner, J. Chem. Inf. Model. 2009, 49, 84-96.
DOI: http://dx.doi.org/10.13171/mjc..5.1/0160113/ferreira
Refbacks
- There are currently no refbacks.
Copyright (c) 2016 Mediterranean Journal of Chemistry
