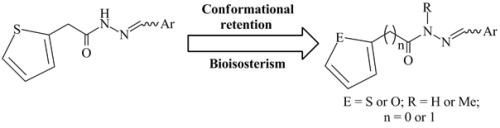
Synthesis and anti-tubercular activity of Thienyl and Furanyl derivatives
Abstract
Full Text:
PDFReferences
I. E. Perepichka, D. F. Perepicka, Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 2 Volume Set, 2009, John Wiley & Sons, Chichester, UK. S. C. Rasmussen, S. J. Evenson, C. B. McCausland. Fluorescent thiophene-based materials and their outlook for emissive applications, Chem. Commun., 2015, 51, 4528-4543.
- L. Chan, O. Pereira, T. J. Reddy, K. D. Sanjov, C. Possion, M. Courchesne, M. Proulx, A. Siddiqui, C. G. Yannopoulos, N. Nguyen-Ba, C. Roy, D. Nasturica, C. Moinet, R. Bethell, M. Hamel, L. L’Heureux, M. David, O. Nicolas, P. Courtrmanche-Asselin, S. Brunette, D. Bilimoria, J. Bedard, Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 2: Tertiary amides, Bioorg. Med. Chem. Lett., 2004, 14, 797-800.
- R. Romagnoli, P. G. Baraldi, P. Cruz-Lopez, M. Tolomeo, A. Di Cristina, R. M. Pipitone, S. Grimaudo, J. Balzarini, A. Brancale, E. Hamei, Synthesis of novel antimitotic agents based on 2-amino-3-aroyl-5-(hetero)arylethynylthiophene derivatives, Bioorg. Med. Chem. Lett., 2011, 21, 2746-2751.
- A. Sivadas, M. P. Satyaseela, T. Bharani, S. K. Upparapalli, N. Subbarava, Design, Synthesis, Characterization and antibacterial activity of methyl -2- (mercaptomethyl)-3-(2-thienyl) acrylate, Int. J. Pharma Sci. Res., 2011, 2, 27-35.
- S. Jain, N. Babu, S. R. Jatti, H. Sahah, S. P. Dhaneira, Synthesis, antitubercular and antifungal activities of heteroaryl-substituted oxiranes derived from Baylis–Hillman adducts, Med. Chem. Res., 2012, 21, 2744-2748.
- S. Saeed, D. N. Rashid, N. Ali, R. Hussain, P. G. Jones, Synthesis, spectroscopic characterization, crystal structure and pharmacological properties of some novel thiophene-thiourea core derivatives, Eur. J. Chem., 2010, 1, 221-227.
- P. R. Kumar, S. Raju, P. S. Goud, M. Sailaja, M. R. Sarma, G. O. Reddy, M. P. Kumar, V. V. R. M. K. Reddy, T. Suresh, P. Hegde, Synthesis and biological evaluation of thiophene [3,2-b] pyrrole derivatives as potential anti-inflammatory agents, Bioorg. Med. Chem., 2004, 12, 1221-1230.
- (a) W. S. Abdel-Aal, H. Y. Hassan, T. Aboul-Fadl, A. F. Youssef. Pharmacophoric model building for antitubercular activity of the individual Schiff bases of small combinatorial library, Europ. J. Med. Chem., 2010, 45, 1098. (b) K. Bedia, O. Elçin, U. Seda, K. Fatma, S. Nathaly, R. Sevim, A. Dimoglo. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity, Europ. J. Med. Chem., 2006, 41, 1253-1261. (c) N. P. Buu-Hoï, N. D. Xuong, N. H. Nam, F. Binon, R. Royer. Tuberculostatic hydrazides and their derivatives, J. Chem. Soc., 1953, 1358-1364. (d) K. M. Thaker, S. D. Tala, B. L. Dodiya, K. A. Joshi, K. L. Dubal, H. S. Joshi. Synthesis of oxadiazoles and pyrazolones as a antimycobacterial and antimicrobial agents, Indian J. Chem., Sect. B, 2011, 50B, 738-744. (e) S. L. Vasoya, M. R. Patel, S. V. Dobaria, H. S. Joshi. Facile synthesis of some new azetidinones and acetyl oxadiazoles bearing benzo[b]thiophene nucleus as a potent biological active agent, Indian J. Chem. Sect. B., 2005, 44B, 405-409. (f) K. M. Thaker, V. V. Kachhadia, H. S. Joshi. Synthesis of 4-thiazolidinones and 2-azetidinones bearing benzo (b) thiophene nucleus as potential antitubercular and antimicrobial agents, Indian J. Chem. Sect B, 2003, 42B, 1544-1547.
- M. V. N. de Souza, M. L. Ferreira, T. C. M. Nogueira, R. S. B. Golçalves, M. A. Peralta; M. S. C. Lourenço, F. R. Vicente, Synthesis and Biological Evaluation of N-(Alkyl)-2-Thiophen-2-Ylacetamides Series As A New Class of Antitubercular Agents, Lett. Drug Des. Discov., 2008, 5, 221-224.
- M. V. N. de Souza, M. C. S. Lourenço, M. A. Peralta, R. S. B. Golçalves, T. C. M. Nogueira, C. H, L. Lima, M. L. Ferreira, E. T. Silva, Synthesis and Biological Evaluation of N,N′-di(thiopheneacetyl)diamines Series as Antitubercular Agents, Phosphorus Sulfur, 2008, 183, 2990-2997.
- M. C. S. Lourenço, F. R. Vicente, M. G. M. O. Henriques, A. L. P. Candea, R. S. B. Golçalves, T. C. M. Nogueira, M. L. Ferreira, M. V. N. de Souza, Synthesis and biological evaluation of N-(aryl)-2-thiophen-2-ylacetamides series as a new class of antitubercular agents, Bioorg. Med. Chem. Lett., 2007, 17, 6895-6898.
- L .N. F. Cardoso, M. L. F. Bispo, C. R. Kaiser, J. L. Wardell, S. M. S. V. Wardell, M. C. S. Lourenço, F. A. F. Bezerra, R. P. P. Soares, M. N. Rocha, M. V. N. de Souza, Anti-Tuberculosis Evaluation and Conformational Study of N-Acylhydrazones Containing the Thiophene Nucleus, Arch. Pharm. Chem. Life Sci., 2014, 347, 432-448.
- A. B. Lopes, E. Miguez, A. E. Kümmerle, V. M. Rumjanek, C. A. M. Fraga, E. J. Barreiro. Characterization of Amide Bond Conformers for a Novel Heterocyclic Template of N-acylhydrazone Derivatives, Molecules, 2013, 18, 11683-11704.
- Y. K. C. da Silva, C. T. M. Reyes, C. G. Rivera, M. A. Alves, E. J. Barreiro, M. S. A. M. Moreira, L. M. Lima, 3-Aminothiophene-2-Acylhydrazones: Non-Toxic, Analgesic and Anti-Inflammatory Lead-Candidates, Molecules, 2014, 19, 8456-8471.
- Z. Cui, Y. Li, Y. Ling, J. Huang, J. Cui, R. Wang, X. Yang, New class of potent antitumor acylhydrazone derivatives containing furan, Eur. J. Med. Chem., 2010, 45, 5576-5584.
- P. Patarski, E. Wyrzykiewicz, G. Bartkowiak, Synthesis and Conformational Assignment of N-(E)-Stilbenyloxymethylenecarbonyl-Substituted Hydrazones of Acetone and o-(m- and p-) Chloro- (nitro-) benzaldehydes by Means of 1H and 13C NMR Spectroscopy, J. Spect., 2013, Article ID 197475, 12 pages.
- V. V. Syakaev, S. N. Podyachev, B. I. Buzykin, S. K. Latypov, W. D. Habicher, I. Konovalov, NMR study of conformation and isomerization of aryl- and heteroarylaldehyde 4-tert-butylphenoxyacetylhydrazones, J. Mol. Struct., 2006, 788, 55-62.
- G. Palla, G. Predieri, P. Domiano, C. Vignali, C. W. Turner, Conformational behaviour andE/Zisomerization of N-acyl andN-aroylhydrazones, Tetrahedron, 1986, 42, 3649-3654.
- (a) I. Warad, S. F. Haddad, M. Al-Noaimi, B. Hammouti, T. Ben Hadda, N'-[(E)-2-Chloro-benzyl-idene]thio-phene-2-carbohydrazide, Acta Crystallogr., Sect E, 2013, 69, 1442; (b) S. Sultan, M. Taha, S. A. A. Shah, B. N. Yamin, H. M. Zaki, (E)-3-Chloro-N′-(2-fluoro¬benzyl¬idene)thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect E, 2014, 70, 751; (c) Y. -L. Zhao, Q. -Z. Zhang, Chen, X.; M. Yu, (E)-N'-[4-(4-Chloro-benz¬yloxy)-3-methoxybenzyl¬idene]thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2006, 62, 5437-5438; (d) J. -H. Jiang, N′-[(5-Methyl-2-fur¬yl)methyl¬ene]thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2010, 66, 922; (e) J.-H. Jiang, N′-(4-Chloro¬benzyl¬idene)thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2010, 66, 923; (f) Y. F. Li, F. F. Jian, N'-(4-Methyl-benzyl-idene)thio-phene-2-carbohydrazide, Acta Crystallogr., Sect E, 2010, 66, 1398; (g) Y. F. Li, F. F. Jian, (E)-N'-(4-Methoxy-benzyl-idene)thio-phene-2-carbohydrazide, Acta Crystallogr., Sect E, 2010, 66, 1400; (h) J. –H. Jiang, N'-Benzyl¬idene¬thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2011, 67, 50; (i) C. –H. Diao, M. Yu, (E)-N'-[3-Meth¬oxy-4-(4-nitro¬benz¬yloxy)benzyl¬idene]thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2006, 62, 5441-5442; (j) Y. F. Li, F. -G. Zhang, F. F. Jian, N'-(4-Cyano-benzyl-idene)thio-phene-2-carbohydrazide, Acta Crystallogr., Sect E, 2010, 66, 1471.
- (a) A. M. Alanazi, S. Lahsasni, A. A. El-Eman, S. W. Ng, N'-[(1E)-(4-Fluoro-phen-yl)methyl-idene]thio-phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2012, 68, 314; (b) A. M. Alanazi, S. Lahsasni, A. A. El-Eman, S. W. Ng, N′-[(1E)-(2,6-Difluoro¬phen¬yl)methyl¬idene]thio¬phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2012, 68, 315.
- (a) H. M. Ali, S. Puvaneswary, W. J. Basirun, S. W. Ng, 3-Hydroxy¬salicylaldehyde 2-thienoylhydrazone, Acta Crystallogr., Sect. E, 2005, 61, 1083-1084; (b) Y. F. Li, J. -H. Jiang, F. -F. Jian, N'-(4-Hy-droxy-benzyl-idene)thio-phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2010, 66, 1719; (c) Z. -L. Jing, Q. -Z. Zhang, M. Yu, X. Chen, N-(2-Hydr¬oxy-3-methoxy¬benzyl¬idene)-N'-(2-thienylcarbonyl)hydrazine monohydrate, Acta Crystallogr., Sect. E, 2006, 62, 4894-4895; (d) J. -H. Jiang, N′-[1-(2-Hy¬droxy¬phen¬yl)ethyl¬idene]thio-phene-2-carbohydrazide, Acta Crystallogr., Sect. E, 2011, 67, 32.
- (a) Y. -F. Li, F. F. Jian, N'-(3,4-Dimethylbenzylidene)furan-2-carbohydrazide, Acta Crystallogr,. Sect. E, 2010, 66, 2061; (b) J. Xu, X. Hue, (E)-4-{[2-(2-Furylcarbonyl)hydrazinylidene]methyl}-2-methoxyphenyl acetate, Acta Crystallogr., Sect. E, 2011, 67, 880; (c) H.-D. Dong, Crystal structure of N'-(5-chloro-2-hydroxybenzylidene)furan-2-carbohydrazide monohydrate, C12H9ClN2O3·H2O, Z. Kristallogr. NCS, 2012, 227, 89-90; (d) J. Xu, N'-(2,6-Dichlorobenzylidene)furan-2-carbohydrazide, Acta Crystallogr., Sect. E, 2012, 68, 1455; (e) H. M. Ali, S. Puvaneswary, W. J. Basirun, S. W. Ng, 3-Hydroxysalicylaldehyde 2-furoylhydrazone, Acta Crystallogr., Sect. E, 2005, 61, 1079-1080; (f) X. -S. Tai, J. Yin, M. -Y. Hao, Z. -P. Liang, (E)-N'-(5-Bromo-2-hydroxybenzylidene)furan-2-carbohydrazide monohydrate, Acta Crystallogr., Sect. E, 2007, 63, 2144-2145.
- (a) Y. -F. Li, F. F. Jian, N'-(4-Cyano-benzyl-idene)furan-2-carbohydrazide monohydrate, Acta Crystallogr., Sect. E, 2010, 66, 1670; (b) Y. -F. Li, F. F. Jian, N'-Benzyl-idene-furan-2-carbohydrazide, Acta Crystallogr., Sect. E, 2010, 66, 1720; (c) R. Bikas, H. H. Monfared, Kazak, C.; N. B. Arslan, K. Bijanzad, (E)-N′-(2-Hy-droxy¬benzyl¬idene)furan-2-carbohydrazide, Acta Crystallogr, Sect. E, 2010, 66, 2015; (d) Y. -F. Li, F. F. Jian, (E)-N'-[4-(Methyl-sulfan-yl)benzyl-idene]furan-2-carbohydrazide monohydrate, Acta Crystallogr., Sect. E, 2010, 66, 2157; (e) Y. -F. Li, F. -Y. Meng, N'-[4-(Dimethyl-amino)-benzyl-idene]furan-2-carbohydrazide, Acta Crystallogr., Sect. E, 2010, 66, 2696; (f) J. -H. Jiang, (E)-N′-(2-Fluoro¬benzyl-idene)furan-2-carbohydrazide, Acta Crystallogr., Sect. E, 2010, 67, 240; (g) A. Sundar, S. Ranjith, G. Rajagopal, N′-[(E)-3-Bromo-5-chloro-2-hy¬droxy¬benzyl¬idene]furan-2-carbohydrazide, Acta Crystallogr., Sect. E, 2014, 70, 670; (h) J. -H. Jiang, N′-(4-Chloro¬benzyl¬idene)furan-2-carbohydrazide monohydrate, Acta Crystallogr., Sect. E, 2010, 66, 627; (i) Y. -L. Zhao, Q. -Z. Zhang, X. Chen, M. Yu, (E)-N'-[4-(4-Chloro¬benz¬yloxy)-3-methoxy¬benzyl¬idene]furan-2-carbohydrazide, Acta Crystallogr., Sect. E, 2007, 63, 2952-2953; (j) X. -S. Tai, J. Yin, F. -Y. Z. Kong, Crystal structure of 2-carboxybenzaldehyde furan-2-carbohydrazide methanol hemisolvate, C13H10N2O4 · 0.5CH3OH, Kristallogr. NCS, 2007, 222, 401-402; (k) Q. -L. Zhou, C. -L. Wang, Z. –L. Jing, 2'-(1,3-Benzodioxol-5-ylmethyl¬ene)furan-2-carbo¬hydrazide, Acta Crystallogr., Sect. E, 2007, 63, 2952.
- R. Bikas, P. M. Anarjan, S. W. Ng, E. R. T. Tiekink, N′-[(E)-2-Hy¬droxy-5-iodo¬benzyl¬idene]furan-2-carbohydrazide monohydrate, Acta Crystallogr, Sect. E, 2012, 68, 413-414.
Refbacks
- There are currently no refbacks.
Copyright (c) 2016 Mediterranean Journal of Chemistry
