Recovery of hexavalent chromium from water using photoactive TiO2-montmorillonite under sunlight
Abstract
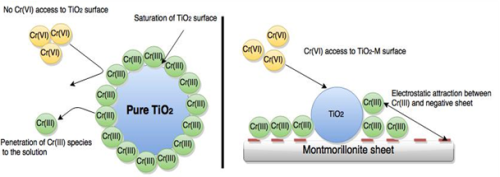
Hexavalent chromium was removed from water under sunlight using a synthesized TiO2-montmorillonite (TiO2-M) employing tartaric acid as a hole scavenger. Cr(VI) species was then reduced to Cr(III) species by electrons arising from TiO2 particles. After that, the produced Cr(III) species was transferred to montmorillonite due to electrostatic attractions leading to set free TiO2 particles for a further Cr(VI) species reduction. Furthermore, produced Cr(III), after Cr(VI) reduction, does not penetrate into the solution. The results indicate that no dark adsorption of Cr(VI) species on TiO2-M is present, however, the reduction of Cr(VI) species under sunlight increased strongly as a function of tartaric acid concentration up to 60 ppm, for which the extent of reduction is maximum within 3 h. On the other hand, the reduction extent of Cr(VI) species is maximum with an initial concentration of Cr(VI) species lower than 30 ppm by the use of 0.2 g/L of TiO2-M. Nevertheless, the increase of the Cr(VI) initial concentration led to increase the amount of Cr(VI) species reduced (capacity of reduction) until a Cr(VI) concentration of 75 and 100 ppm, for which it remained constant at around 221 mg/g. For comparison, the increase of Cr(VI) species concentration in the case of the commercial TiO2 P25 under the same conditions exhibited its deactivation when the reduced amount decreased from 198.1 to 157.6 mg/g as the concentration increased from 75 to 100 ppm.
Full Text:
PDFRefbacks
- There are currently no refbacks.
Copyright (c) 2016 Mediterranean Journal of Chemistry
