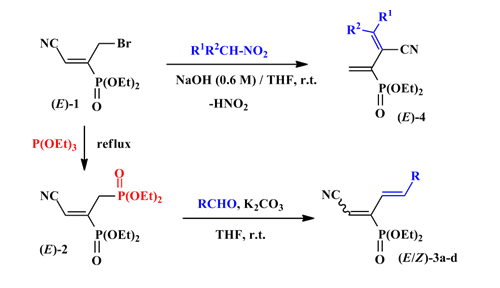
Highly stereoselective synthesis of functionalized 1,3-dienes from a new allyl bromide
Abstract
Full Text:
PDFReferences
- (a) B. Tarnchompoo, C. Thebtaranonth, Y. Thebtaranonth, Tetrahedron Lett. 1987, 28, 6671–6674; (b) B. Tarnchompoo, C. Thebtaranonth, Y. Thebtaranonth, Tetrahedron Lett. 1987, 28, 6675-6678.
- M. Kotera, J. M. Lehn, J. P. Vigneron, Tetrahedron 1995, 51, 1953-1972.
- W. J. Bailey, R. L. Hudson, E. T. Yates, J. Org. Chem. 1963, 28, 828-831.
- (a) Z. Rappoport, The chemistry of dienes and polyenes; Vol. 1. Chichester: John Wiley & Sons 1997; (b) Z. Rappoport, The chemistry of dienes and polyenes; Vol. 2. Chichester: John Wiley & Sons 2001.
- X. Zhang, R. C. Larock, Org. Lett. 2003, 5, 2993-2996.
- C. C. Yu, D. K. P. Ng, B. L. Chen, T. Y. Luh, Organometallics 1994, 13, 1487-1497.
- B. E. Sime, B. Rickborn, J. M. Flournoy, I. B. Berlman, J. Org. Chem. 1988, 53, 4613-4616.
- (a) E. I. Negishi, Z. Huang, G. Wang, S. Mohan, C. Wang, H. Hattori, Acc. Chem. Res. 2008, 41, 1474-1485; (b) J. S. Glasby, Encyclopaedia of the Terpenoids, Wiley, Chichester, UK, 1982; (c) T. K. Devon, A. I. Scott, Handbook of Naturally Occurring Compounds, Vol. 2, Academic, New York, 1972; (d) M. DellaGreca, C. D. Marino, A. Zarrelli, B. D’Abrosca, J. Nat. Prod. 2004, 67, 1492–1495; (e) F. Lv, Z. Deng, J. Li, H. Fu, R. W. M. van Soest, P. Proksc, W. Lin, J. Nat. Prod. 2004, 67, 2033-2036; (f) Y. Cheng, B. Schneider, U. Riese, B. Schubert, Z. Li, M. Hamburger, J. Nat. Prod. 2004, 67, 1854–1858; (g) E. W. Rogers, T. F. Molinski, J. Nat. Prod. 2005, 68, 450-452; (h) H. Mohamad, N. H. Lajis, F. Abas, A. M. Ali, M. A. Sukari, H. Kikuzaki, N. Nakatani, J. Nat. Prod. 2005, 68, 285-288.
- A. –R. Han, M. –S. Kim, Y. H. Jeong, S. K. Lee, E. K. Seo, Chem. Pharm. Bull. 2005, 53, 1466-1468.
- A. R. Pereira, J. A. Cabezas, J. Org. Chem. 2005, 70, 2594-2597.
- J. A. Moreira, J. S. McElfresh, J. G. Millar, J. Chem. Ecol. 2006, 32, 169-194.
- R. M. de Figueiredo, R. Berner, J. Julis, T. Liu, D. Turp, M. Christmann, J. Org. Chem. 2007, 72, 640-642.
- M. Adeva, H. Sahagun, E. Caballero, R. Pelaez-Lamamie de Clairac, M. Medarde, F. Tome, J. Org. Chem. 2000, 65, 3387-3394.
- M. Adeva, F. Buono, E. Caballero, M. Medarde, F. Tome, Synlett 2000, 832-834.
- D. Amans, V. Bellosta, J. Cossy, Chem. Eur. J. 2009, 15, 3457-3473.
- G. Ramamoorthy, C. M. Acevedo, E. Alvira, M. A. Lipton, Tetrahedron: Asymmetry 2008, 19, 2546-2554.
- M. Boldi, Curr. Opin. Chem. Biol. 2004, 8, 281-286.
- Y. Liao, Y. –H. Hu, J. Wu, Q. Zhu, M. Donovan, R. Fathi, Z. Yang, Curr Med. Chem. 2003, 10, 2285-2316.
- M. Kalesse, M. Christmann, Synthesis 2002, 981-1003.
- (a) O. Diels, K. Alder, Liebigs Ann. Chem. 1928, 460, 98–122; (b) F. Fringuelli, A. Taticchi, The Diels-Alder Reaction: Selected Practical Methods, West Sussex, England: John Wiley & Sons 2002, 340; (c) J. Sauer, R. Sustmann, Angew. Chem., Int. Ed. Engl. 1980, 19, 779-807; (d) S. Reymond, J. Cossy, Chem. Rev. 2008, 108, 5359-5406.
- K. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem., Int. Ed., 2002, 41, 1668-1698.
- T. J. Brocksom, A. G. Correa, R. M. Naves, F. Silva, V. Catani, M. A. Ceschi, J. Zukerman-Schpector, A. P. Toloi, M. L. Ferreira, U. Brocksom, Org. Synth. Theory Appl. 2001, 5, 39-87.
- S. Fustero, P. Bello, B. Fernandez, C. Del Pozo, G. B. Hammond, J. Org. Chem. 2009, 74, 7690-7696.
- J. McNulty, P. Das, Tetrahedron Lett. 2009, 50, 5737-5740.
- S. Laclef, C. J. Exner, M. Turks, V. Videtta, P. Vogel, J. Org. Chem. 2009, 74, 8882-8885.
- L. J. Perez, H. L. Shimp, G. C. Micalizio, J. Org. Chem. 2009, 74, 7211-7219.
- X. Huang, T. de Haro, C. Nevado, Chem. Eur. J. 2009, 15, 5904-5908.
- H. –J. Zhang, Z. Song, C. Wang, C. Bruneau, Z. Xi, Tetrahedron Lett. 2008, 49, 624-627.
- M. Ogasawara, H. Murakami, T. Furukawa, T. Takahashi, N. Shibata, Chem. Commun. 2009, 7366-7368.
- U. UrÅ¡iÄ, U. GroÅ¡elji, A. Meden, J. Svete, B. Stanovnik, Tetrahedron Lett. 2008, 49, 3775-3778.
- D. P. J. Hamon, P. R. Spurr, Synthesis 1981, 873-879.
- R. M. Davidson, G. L. Kenyon, J. Org. Chem. 1977, 42, 1030-1035.
- M. F. Semmelhack, P. Helquist, L. D. Jones, L. Keller, L. Mendelson, L. S. Ryono, J. G. Smith, R. D. Stauffer, J. Am. Chem. Soc. 1981, 103, 6460-6471.
- R. L. Cobb, J. E. Mahan, J. Org. Chem. 1977, 42, 2829-2832.
- D. Bellus, C. D. Weis, Tetrahedron Lett. 1973, 14, 999-1000.
- D. Bellus, K. Von Bredow, H. Sauter, C. D. Weis, Helv. Chim. Acta 1973, 56, 3004-3038.
- M. G. Pettett, A. B. Holmes, J. Chem. Soc., Perkin Trans. 1 1985, 1161-1165.
- H. Kiji, T. Okano, E. Fujii, J. Tsuji, Synthesis 1997, 869-870.
- A. Fray, J. Ben Kraïem, A. Souizi, H. Amri, Arkivoc 2012, viii, 119-127.
- A. Fray, J. Ben Kraïem, H. Amri, Heteroatom Chem. 2013, 24, 460–465.
- A. Fray, J. Ben Kraïem, A. Arfaoui, H. Amri, Sci.World J. 2014, 2014, Article ID 260325.
- (a) F. Béji, J. Lebreton, J. Villiéras, H. Amri, Tetrahedron 2001, 57, 9959-9962; (b) F. Béji, J. Lebreton, J. Villiéras, H. Amri, Synth. Commun. 2002, 32, 3273-3278.
- H. Kraïem, H. Amri, Phosphorus, Sulfur and Silicon 2007, 182, 2555-2564.
- M. Ameur, A. Arfaoui, J. Ben Kraïem, S. S. Al-Deyab, H. Amri, Med. J. Chem. 2012, 2, 347-354.
- T. Janecki, R. Bodalsky, Synthesis 1990, 799-801.
- D. Basavaiah, S. Pandiaraju, Tetrahedron 1996, 52, 2261-2268.
- V. K. Brel, E. V. Abramkin, Zh. Obshch. Khim. 1994, 64, 1989-1994.
DOI: http://dx.doi.org/10.13171/mjc.3.3.2014.12.06.01
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
