Synthesis and biological evaluation of new pyrazolo[3,4-d]pyrimidine derivatives
Abstract
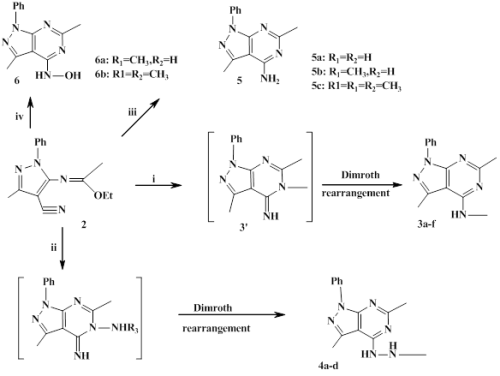
Several new pyrazolopyrimidine compounds were achieved from aminocyanopyarazole 1. The starting material 1 was initially coupled with orthoester at refluxed with various primary amines, ammonia, hydrazines and hydroxylamine to furnish a series of pyrazolo[3,4-d]pyrimidines. The reaction of imidate 2a-b with hydrazide derivatives led to the formation of pyrazolo[3,4-d][1,2,4]triazolo[4,3-c]pyrimidines. Some of the synthesized compounds 3a and 4c were evaluated for their anti-inflammatory, antipyretic and nociceptive activities. We start by studing the toxicity of these two molecules by measuring the corresponding DL50. The DL50 of 3a and 4c are estimated to 1333.2mg / kg and 1593.5mg / kg respectively. Pharmacological evaluation showed that compounds 3a and 4c at doses (5.5-22.2 mg / Kg, i.p) exhibited anti-inflammatory activities compared to Ibuprofen (150 mg / Kg, i.p), used as a refer ence drug. Further, our study showed that the injection of derived pyrazolopyrimidines on hyperthermic animal leads to a decrease in temperature after 1 hours of treatment compared to paracetamol used as reference. In addition, the injection of derived pyrazolopyrimidines at different doses contains a potent nociceptive activity. This effect is dose-dependent compared to aspirin.
Full Text:
PDFReferences
- M. B. Tollefson,.B. A Acker, E. J. Jacobsen, R. O. Hughes, J. K.Walker, D. N. A. Fox, M. J. Palmer, S. K. Free- man, Y. Yu, Bond, Bioorg. Med. Chem. Lett. 2010, 20 (10), 3120.
- A. A. Aly, I. A. G. El-Karim, Journal of the Korean Chemical Society 2011, 55:5. doi.org/10.5012/jkcs.2011.55.5.7813.
- A. V. Ivachtchenko, D. E. Dmitriev, E. S. Golovina, E. S. Dubrovskaya,; M. G. Kadieva, A. G. Koryakova, V. M. Kysil, O. D. Mitkin, S. E. Tkachenko, I. M. Okun, A. A. Vorobiov, Bioorg. Med. Chem. Lett. 2010, 20(7), 2133.
- M. Bakavoli, G. Bagherzadeh, M. Vaseghifar, A. Shiri, M. Pordel, M. Mashreghi,; P. Pordeli, M. Araghi, Eur. J. Med. Chem. 2010, 4(2), 647.
- K. J. Curran, J. C. Verheijen, , J. Kaplan, D. J. Richard, L. Toral-Barza, I. Hollander, J. Lucas, S. Ayral-Kaloustian, K. Yu, A. Zask, Bioorg. Med. Chem. Lett. 2010, 20(4), 1440.
- I. Kim, J. H. Song, C. M. Park,; J. W. Jeong, H. R. Kim, J. R. Ha, Z. No,; Y. L. Hyun, Y. S. Cho, N. S. Kang, D. J. Jeon, Bioorg. Med. Chem. Lett. 2010, 20(3), 922.
- M. Bakavoli, G. Bagherzadeh, M. Vaseghifar, A. Shiri, M. Pordel, M. Mashreghi, P. Pordeli, M. Araghi, Eur. J. Med. Chem. 2010, 45(2), 647.
- L. Yuan, C. W. Song, C. Li, Y. Li, L. Dong, S.Yin, European Journal of Medicinal Chemistry 2013, 67:152-157.
- S. Schenone, C. Brullo, O. Bruno, F. Bondavalli, L. Mosti, G. Maga, E. Crespan, F. Carraro, F. Manetti, C. Tin- tori, M. Botta, Eur. J. Med. Chem. 2008, 43(12), 2665.
- J. D. Anderson, H. B. Cottam, S. B. Larson, L. D. Nord, G. R. Revankar, R. K. Robins, J. Heterocycl. Chem. 1990, 27, 439.
- A. Aggarwal, V. Kumar, R.; Kumar, S. P. Singh Beilstein, J. Org. Chem. 2011, 7, 179–197.
- A. Karoui, F. Allouche, M. Deghrigue, A. Agrebi, A. Bouraoui, F. Chabchoub, Med Chem Res. 2014, 23,1591-1598.
- H. Tamta, S. Kalra, A. K. Mukhopadhyay, Biochemistry (Moscow). 2006, 71, S49-S54.
- A.E. Rachad, M. I. Hegab, R. E. Abdel-Megeid, F. M. E. Abde-Megeid, Bioorg. Eur. J. Med. Chem. 2009, 44, 3285-3292.
- A. S. Shawali, H. M. Hassaneen, N. Kh. Shurrab, Tetrahedron , 64, 10339-10343.
- R. Koster, M. Anderson, E. J. Beer, Federation Proceeds, 1959, 18, 412–416.
- C. A. Winter, E. A. Risley, , G.W. Nuss, Prot. Soc. Exper. Biol. Med. 1962, 111, 544- 547.
- O. A. Olajide, J. M. Makinde, D. T. Okpako, S. O. Awe, Journal of Ethnopharmacology. 2000, 71, 153-160.
- M.S. Al-Ghamdi, Journal of Ethnopharmacology. 2001, 76, 45-48.
DOI: http://dx.doi.org/10.13171/mjc.3.2.2014.13.05.23agrebi
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
