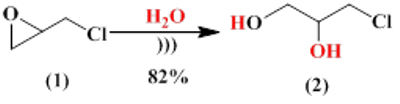
Eco-friendly and highly efficient multigram synthesis of 3-chloro-1,2-propanediol using sonochemistry
Abstract
Full Text:
PDFReferences
- A. Padwa and S. S. Murphree, Epoxides and aziridines - A mini review, Arkivoc, 2006, iii, 6-33.
- G.W. Ware, Epichlorohydrin, Reviews of Environmental Contamination and Toxicology, 1989, 107, 65-77.
- S. J. Vali, R. B. Ganduri and S. S. Sait, Estimation of epichlorohydrin content in pharmaceutical drug substances by capillary gas chromatography with flame ionisation detection, Journal of Chemical and Pharmaceutical Research, 2011, 3, 392-399.
- B. M. Bell et al., Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process, Clean: Soil, Air, Water, 2008, 36, 657-661.
- V. Mirkhani, S. Tangestaninejad, B. Yadollahi and L. Alipanah, Efficient regio- and stereoselective ring opening of epoxides with alcohols, acetic acid and water catalyzed by ammonium decatungstocerate (IV), Tetrahedron, 2003, 59, 8213-8218.
- M. Moghadam, S. Tangestaninejad, V. Mirkhani and R. Shaibani, Rapid and efficient ring opening of epoxides catalyzed by a new electron deficient tin (IV) porphyrin, Tetrahedron, 2004, 60, 6105-6111.
- W. X. Zhang and K. Ye, Hydrolysis of epoxides and aziridines catalyzed by polymer-supported quarternary ammonium bisulfate, Chinese Chemical Letters, 2008, 19, 146-148.
- B. Li et al., Hydration of Epoxides on [CoIII(salen)] Encapsulated in Silica-Based Nanoreactors, Angewandte Chemie International Edition, 2012, 51, 11517-11521.
- W. M. Ren, Y. M. Wang, R. Zhang, J. Y. Jiang and X. B. Lu, Mechanistic Aspects of Metal Valence Change in SalenCo(III)OAc-Catalyzed Hydrolytic Kinetic Resolution of Racemic Epoxides, The Journal of Organic Chemistry, 2013, 78, 4801-4810.
- S. Bonollo, D. Lanari and L. Vaccaro, Ring-Opening of Epoxides in Water, European Journal of Organic Chemistry, 2011, 14, 2587-2598.
- Z. Wang, Y. Cui, Z. Xu and J. Qu, Hot Water-Promoted Ring-Opening of Epoxides and Aziridines by Water and Other Nucleopliles, The Journal of Organic Chemistry, 2008, 73, 2270-2274.
- Y. Yang, W. Wang, A. Wumaier, R. Sheng, X. Zhang and T. Zhang, Practical and efficient utilisation of (R)-3-chloro-1,2-propanediol in synthesis of L-carnitine, Journal of Chemical Research, 2011, 35, 371-372.
- M. R. Barbachyn and C. W. Ford, Oxazolidinone structure-activity relationships leading to linezolid, Angewandte Chemie International Edition, 2003, 42, 2010-2023.
- Z. Y. WANG, Y. WANG, L. W. SUN and J. T. ZHU, Asymmetric Synthesis of (R)- and (S)-Moprolol, Chemical Research in Chinese Universities, 2008, 24, 747-751.
- X. Q. Li et al., Asymmetric synthesis of L-carnitine from (R)-3-chloro-1,2-propanediol, Chinese Chemical Letters, 2011, 22, 765-767.
- J. Chengjun and H. Huabin, A New Practical Synthesis of Ethyl (R)-(-)-4-Cyano-3-hydroxybutyrate From (S)-3-chloro-1,2-propanediol, Letters in Organic Chemistry, 2012, 9, 520-521.
- P. J. Dunn, The importance of Green Chemistry in Process Research and Development, Chemical Society Reviews, 2012, 41, 1452-1461.
- R. Dua, S. Shrivastava, S. L. Shrivastava and S. K. Srivastava, Green Chemistry and Environmentally Friendly Technologies: A Review, Middle-East Journal of Scientific Research, 2012, 11, 846-855.
- L. S. S. Pinto, E. T. da Silva and M. V. N. de Souza, A Scalable and Efficient Synthesis of 3-Chloro-1,2-propanediol, Organic Preparations and Procedures International: The New Journal for Organic Synthesis, 2016, 48, 319-320.
DOI: http://dx.doi.org/10.13171/mjc56/01607231833/desouza
Refbacks
- There are currently no refbacks.
Copyright (c) 2016 Mediterranean Journal of Chemistry
