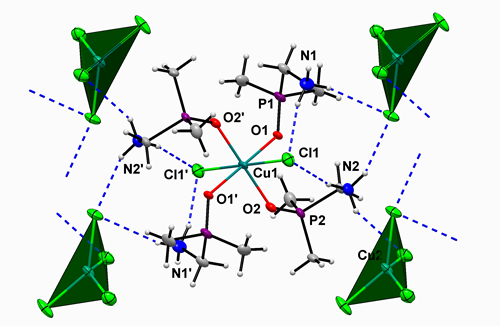
Synthesis, Spectroscopy and Crystal Structure of a New Copper Complex Built Up by Cationic (Dimethylphosphoryl)methanaminium Ligands
Abstract
Full Text:
PDFReferences
- Brunet, P., Simard, M., Wuest, J. D., J. Am. Chem. Soc., 1997, 119, 2737-2738.
- Hosseini, M. W., Acc. Chem. Res., 2005, 38, 313-323.
- Buhl, D., Gün, H., Jablonka, A., Reiss, G. J., Crystals, 2013, 3, 350-362.
- Czaikowsky, D., Davidov, A., Reiss, G. J., Z. Kristallogr. New Cryst. Struct., 2014, 229, 29-30.
- Lambertz, C., Luppa, A., Reiss, G. J., Z. Kristallogr. New Cryst. Struct., 2013, 228, 227-228.
- Reiss, G. J., Jörgens, S., Acta Cryst., 2012, E68, o2899-o2900.
- Reiss, G. J., Acta Cryst., 2013, E69, o1253-o1254.
- Reiss, G. J., Acta Cryst., 2013, E69, m614-m615.
- Bianga C. M., Eggeling, J., Reiss, G. J., Acta Cryst., 2013, E69, o1639-o1640.
- Reiss, G. J., Z. Kristallogr. New Cryst. Struct., 2013, 228, 431-433.
- Borisov, G., Varbanov, S. G., Venanzi, L. M., Albinati, A., Demartin, F., Inorg. Chem., 1994, 33, 5430-5437.
- Dodoff, N., Macicek, J., Angelova, O., Varbanov, S. G. & Spassovska, N., J. Coord. Chem., 1990, 22, 219-228.
- Kochel, A., Inorg. Chim. Acta, 2009, 362, 1379-1872.
- Reiss, G. J., Acta Cryst., 2013, E69, m248-m249.
- Reiss, G. J., Acta Cryst., 2013, E69, m250-m251.
- Ershov, M. A., Skvortsov, V. G., Pilchikova, Y. Y., Koltsova, O. V., Suponitsky, K. Y., Acta Cryst. 2006, E62, m3076-m3077
- Girma, K. B., Lorenz, V., Blaurock, S., Edelmann, F. T., Inorg. Chim. Acta, 2006, 359, 364-368.
- Reiss, G. J., Helmbrecht, C. Acta Cryst., 2012, E68, m1402-m1403 and references cited there.
- Grell, J., Bernstein, J., Tinhofer, G., Acta Cryst., 1999, B55, 1030-1043.
- Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Version 3.2i. Crystal Impact, Bonn, Germany 2012
- Agilent Technologies: CrysAlis PRO Software system, version 1.171.35.15, Agilent Technologies UK Ltd, Oxford, UK 2011.
- Sheldrick, G. M., Acta Cryst., 2008, A64, 112-122.
DOI: http://dx.doi.org/10.13171/mjc.3.2.2014.01.05.19
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
