First Description of a Guanidine-embedded Pillar[5]arene: Opening New Avenues for Biological Applications
Abstract
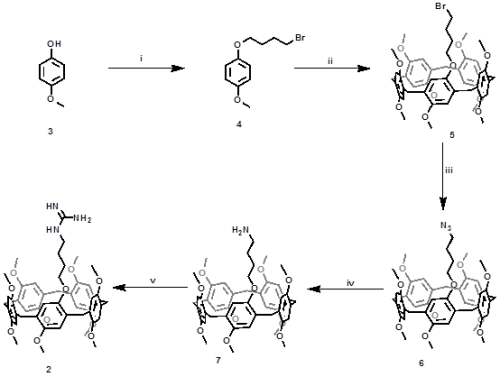
Full Text:
PDFReferences
- (a) H. Oediger, K. Eiter, F. Möller, Synthesis, 1972,591-598; (b) J.E. Taylor, S.D. Bull, J.M.J. Williams, Chem. Soc. Rev., 2012, 41, 2109-2121.
- (a) C. Alonso-Moreno, A. Antiñolo, F. Carrillo-Hermosilla, A. Otero, Chem. Soc. Rev., 2014, 43, 3406-3425 (b) C. Savelli, R. Salvio, Chem.-Eur. J. 2015, 21, 5856-5863.
- (a) T. Ishikawa, T. Isobe, Chem.Eur. J., 2002, 8, 553-557;(b) D. Leow, D.; C.H. Tan, Chem. Asian J., 2009, 4, 488-507;(c) P. Selig, Synthesis, 2013, 703-718.
- M.P. Coles, Dalton Trans., 2006, 985-1001.
- T. Yamada, X. Liu, U. Englert, U.; H. Yamane, Dronskowski, Chem.Eur. J., 2009, 15, 5651-5655.
- F. Sansone, M. DudiÄ, G. Donofrio, C. Rivetti, L. Baldini, A. Casnati, S. Cellai, R. Ungaro, J. Am. Chem. Soc., 2006, 128, 14528-14536.
- V. Bagnacani, V. Franceschi, M. Bassi, M. Lomazzi, G. Donofrio, F. Sansone, A. Casnati, R. Ungaro, Nat. Commun. 4:1721, doi:10.1038/ncomms2721 2013 Nat. Commun. | www.nature.com/naturecommunications.
- V. Martos, S. C. Bell, E. Santos, E.Y. Isacoff, D. Trauner, D.; J. de Mendoza, Proc. Natl. Acad. Sci. 2009, 106, 10482-10486.
- (a)T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022; (b) P.J. Cragg, K. Sharma, Chem. Soc. Rev., 2012,41, 597-607 (c) S. Cuhawijay, R.R. Shaikh, H. Qian, Y. Zhang, K. Meguellati, Y.W. Yang, Chem. Commun. 2017, 53, 677-696.
- (a) T.G. Ong, J.S. O'Brien, I. Korobkov, D.S. Richeson, Organometallics 2006, 25, 4728-4730; (b) H. Shen, H.S. Chan, Z. Xie, Organometallics 2006, 25, 5515-5517; (c) J. Li, Z. Zhang, E. Fan, Tetrahedron Lett. 2004, 45, 1267-1269; (d) Y.Q. Wu, S.K. Hamilton, D.E. Wilkinson, G.S. Hamilton, J. Org. Chem. 2002, 67, 7553-7556; (e) G. Evindar, R.A. Batey, Org. Lett. 2003, 5, 133-136; (f) D.A. Powell, P.D. Ramsden, R.A. Batey, J. Org. Chem. 2003, 68, 2300-2309; (g) B.R. Linton, A.J. Carr, B.P. Orner, A.D. Hamilton, J. Org. Chem. 2000, 65, 1566-1568; (h) M.K.T. Tin, N. Thirupathi, G.P.A. Yap, D.S. Richeson, Dalton Trans. 1999, 17, 2947-2950; (i) K. Feichtinger, C. Zapf, H.L. Sings, M. Goodman, J. Org. Chem. 1998, 63, 3804-3805.
- (a) M.A. Poss, E. Iwanowicz, J.A. Reid, J. Lin, Z. Gu, Tetrahedron Lett. 1992, 33, 5933-5936; (b) S.E. Schneider, P.A. Bishop, M.A. Salazar, O.A. Bishop, E.V. Anslyn, Tetrahedron 1998, 54, 15063-15086.
DOI: http://dx.doi.org/10.13171/mjc62/01701171722-meguellati
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
