Controlled release of targeted anti-leukemia drugs azacitidine and decitabine monitored using surface-enhanced Raman scattering (SERS) spectroscopy
Abstract
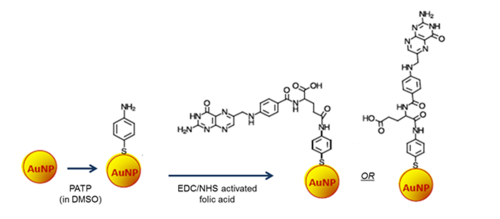
A new targeted drug delivery system with controlled release of anti-cancer drugs, azacitidine and decitabine, was investigated to enhance the efficacy of cancer treatment and reduce the effects of high drug toxicity to healthy tissues. The proposed drug nanocarriers are based on gold nanoparticles (AuNPs) modified with mercaptobenzoic acid (MBA) linker to enable the immobilization of azacitidine (AZA) and decitabine (DAC) on AuNPs in the form of AuNP@MBA/AZA,DAC entities. The cancer cell recognition was accomplished by covalently binding folic acid (FA) ligands to para-aminothiophenol (PATP) in the mixed SAM shell on gold nanoparticle nanocarriers, AuNP@MBA,PATP. The FA ligand was used due to the strong expression of folic acid receptors (FR) in the membrane of cancer cells. This enables the functionalized carriers to target only cancer cells owing to the efficient FA-FR binding property. The amide bonds between the linkers and azacitidine/decitabine are pH sensitive and undergo acid hydrolysis in a low pH environment of the cytosol in cancer cells. Using the solutions of different pH, the release of azacitidine/decitabine was monitored by surface-enhanced Raman scattering spectroscopy (SERS) measurements of the MBA Raman modes at 1586 cm-1 and 1074 cm-1 . At pH 7.4, the release of the drug was found to be negligible, while at pH 4.0 and 5.5 a continuous drug release was observed over 3 hours. The utilization of SERS monitoring for the drug release was based on the strong Raman signals which are generated by the MBA linker when it is bound to a plasmonic AuNP. During the immobilization of azacitidine/decitabine on AuNP carriers, the SERS signals are strongly reduced due to the shielding by drug molecules but they increase sharply upon the drug release confirming the amide bond breakage and successful drug delivery.
Full Text:
PDFReferences
- D. Bobo, K.J. Robinson, J. Islam, K.J. Thurecht and S.R. Corrie, Pharm. Res., 2016, 33, 2373-2387.
- H. Ilkhani, T. Hughes, J. Li, C.J. Zhong and M. Hepel, Biosens. Bioelectron., 2016, 80, 257-264.
- J. Li, Z. Skeete, S. Shan, S. Yan, K. Kurzatkowska, W. Zhao, Q.M. Ngo, P. Holubovska, J. Luo, M. Hepel and C.J. Zhong, Anal. Chem., 2015, 87, 10698−10702.
- K. Kurzatkowska, T. Santiago and M. Hepel, Biosens. Bioelectron., 2017, 91, 780-787.
- S. Luo, X. Yang and C. Shi, Curr. Med. Chem., 2016, 23, 483-497.
- H. Sharma, P.K. Mishra, S. Talegaonkar and B. Vaidya, Drug Discov. Today, 2015, 20, 1143-1151.
- Y. Ding, Z. Jiang, K. Saha, C.S. Kim, S.T. Kim, R.F. Landis and V.M. Rotello, Mol. Therapy, 2014, 22, 1075-1083.
- R. Hong, G. Han, J.M. Fernández, B.J. Kim, N.S. Forbes and V.M. Rotello, J. Am. Chem. Soc., 2006, 128, 1078-1079.
- P.T. Wong and S.K. Choi, Chem Rev., 2015, 115, 3388-3432.
- P. Fenaux, Nat. Clin. Prac. Oncol., 2005, 12, S36-S44.
- P. Fenaux, G.J. Mufti, E. Hellstrom-Lindberg and e. al., Lancet Oncol., 2009, 10, 223-232.
- J.E. Bilbault, N. Cambier, J.M. Lemahieu, B. Quesnel, M. Aufret and C. Rose, J. Clin. Oncol, 2011, 29, e411-e412.
- E.J.B. Derissen, J.H. Beijnen and J.H.M. Schellens, Oncologist, 2013, 18, 619-624.
- S.K. Sadashiv, C. Hilton, C. Khan, J.M. Rossetti, H.L. Benjamin, S. Fazal, E. Sahovic, R.K. Shadduck and J. Lister, Cancer Med., 2014, 1570-1578.
- A. Pulsoni, L. Pagano, R. Latagliata, M. Casini, R. Cerri, M. Crugnola and e. al., Haematologica, 2004, 89, 296-302.
- J. Aimiuwu, H. Wang, P. Chen, Z. Xie, J. Wang, S. Liu and e. al., Blood, 2012, 119, 5229-5238.
- K. Raj and G.J. Mufti, Ther. Clin. Risk Manag., 2006, 2, 377-388.
- A. Latorre, P. Couleaud, A. Aires, A.L. Cortajarena and A. Somoza, Eur. J. Med. Chem., 2014, 82, 355-362.
- J. Sudimack and R.J. Lee, Adv. Drug Deliv. Rev., 2000, 41, 147-162.
- C. Streseman and F. Lyko, Int. J. Cancer., 2008, 123, 8-13.
- S.C. Navada, J. Steinmann, M. Lübbert and L.R. Silverman, J. Clin. Investig., 2014, 124, 4-46.
- D.V. Santi, A. Norment and C.E. Garrett, Proc. Natl. Acad. Sci USA, 1984, 81, 6993-6197.
- L. Chen, A.M. MacMillan, W. Chang, K. Ezaz-Nikpay, W.S. Lane and G.L. Verdine, Biochem., 1991, 30, 11018-11025.
- M. Schaefer, S. Hagemann, K. Hanna and F. Lyko, Cancer Res., 2009, 69, 8127-8132.
- M.J. Dapp, C.I. Clouser, S. Patterson and L.M. Mansky, J. Virology, 2009, 83, 11950-11958.
- R.C. Lynn, M. Poussin, A. Kalota, Y. Feng, P.S. Low, D.S. Dimitrov and D.J. Powell, Blood, 2015, 125, 3466-3476.
- G.J. Roboz, Curr. Opin. Oncol., 2012, 24, 711-719.
- A.S. Wibowo, M. Singh, K.M. Reeder, J.J. Carter, A.R. Kovach, W. Meng, M. Ratnam, F. Zhang and C.E.D. III, Proc. Natl. Acad. Sci. USA, 2013, 110, 15180-11518.
- W. Xia and P.S. Low, J. Med. Chem., 2010, 53, 6811-6824.
- C.P. Leamon and A.L. Jackman, Vitam. Horm., 2008, 79, 203-233.
- H. Elnakat and M. Ratnam, Adv. Drug. Deliv. Rev., 2004, 56, 1067-1084.
- M. Stobiecka, K. Coopersmith and M. Hepel, J. Colloid Interface Sci, 2010, 350, 168-177.
- M. Stobiecka and M. Hepel, Phys. Chem. Chem. Phys., 2011, 13, 1131-1139.
- M. Stobiecka and M. Hepel, Biomaterials, 2011, 32, 3312-3321.
- M. Stobiecka and A. Chalupa, J. Phys. Chem. B, 2015, 119, 13227-13235.
- M. Hepel and M. Stobiecka, in Fine Particles in Medicine and Pharmacy; ed. E. Matijevic; Springer Sci Publ.: New York, 2012, pp. 241-281.
DOI: http://dx.doi.org/10.13171/mjc64/01706081223-hepel
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
