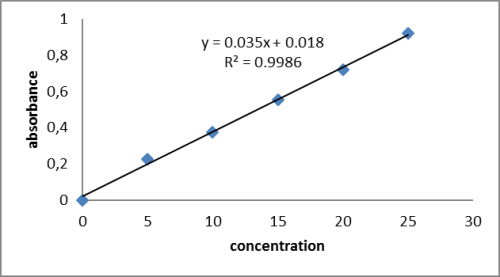
Development and Validation of UV Spectrophotometric Method For Determination of Bisoprolol Fumarate in Bulk and Pharmaceutical Dosage Forms
Abstract
Full Text:
PDFReferences
T.F. Ligenli, F. Kilicaslan, A. Kirilmaz, M. Uzun, Bisoprolol improves echocardiographic parameters of left ventricular diastolic function in patients with systemic hypertension, Cardiology, 2006, 106, 127-131.
J.K. Mc Gavin, G.M. Keating, Bisoprolol: A review of its use in chronic heart failure, Drugs, 2002, 62, 267-269.
R.N. Goyal, A. Tyagi, N. Bachheti, S. Bishnoi, Voltammetric determination of bisoprolol fumarate in pharmaceutical formulations and urine using single-wall carbon nanotubes modified glassy carbon electrode, Electrochimica Acta, 2008, 53, 2802-2808
R. Sahu, V.B. Patel, Simultaneous spectrophotometric estimation of hydrochlorothiazide and bisoprolol fumarate in combined dosage forms, Indian Journal of Pharmaceutical Sciences, 2006, 68, 764 - 767
A.D. Panainte, N. Bibire, G. Tantaru, M. Apostu, M. Vieriu, and V. Dorneanu, A new method for the assay of bisoprolol using bromocresol green. Rev. Chim. (Bucharest), 2014, 65(8), 916-920.
A.D. Panainte, N. Bibire, G. Tantaru, M. Apostu, M. Vieriu, and V. Dorneanu, Spectrophotometric method for the estimation of bisprolol fumarate in tablets, Rev. Med. Chir. Soc. Med. Nat lasi., 2014, 118 (2), 558-563.
A.D. Gudruman, A. Murarasu, and V. Dorneanu, Spectrophotometric determination of bisoprolol using methyl orange as reagent. Farmacia, 2012, 60(5), 634-640
D.N. Vora, A.A. Kadav, Development and validation of a simultaneous HPLC method for estimation of bisoprolol fumarate and amlodipine besylate from tablets, Indian Journal of Pharmaceutical Sciences, 2008, 70, 542-546
S. Shaikh, O.A. Thusleem, M.S. Muneera, J. Akmal, A.V. Kondaguli, K. Ruckmani, A simple and rapid high-performance liquid chromatographic method for the determination of bisoprolol fumarate and hydrochlorothiazide in a tablet dosage form, Journal of Pharmaceutical and Biomedical Analysis, 2008, 48, 1055-1057.
E. Caudron, S. Laurent, E.M. Billaud, P. Prognon, Simultaneous determination of the acid/base antihypertensive drugs celiprolol, bisoprolol and irbesartan in human plasma by liquid chromatography, Journal of Chromatography B, 2004, 801, 339-345.
C. Oniscu, C.V. Vlase, G. Peste, A new high-performance liquid chromatographic method for determination of bisoprolol in plasma samples, Romanian Biotechnological Letters, 2007, 12, 3079-3084.
K. Konam, J. Soujanya, M. Sasikala, and A.K. Kumar, Development and validation of RP-HPLC method for the determination of bisoprolol fumarate tablets. International Journal of Research in Pharmaceutical and Nano Sciences, 2013, 2(1), 57-58.
ICH G. Validation of analytical procedures: text and methodology. Q2 (R1). 2005;1.
DOI: http://dx.doi.org/10.13171/mjc65/01710181149-shantier
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
