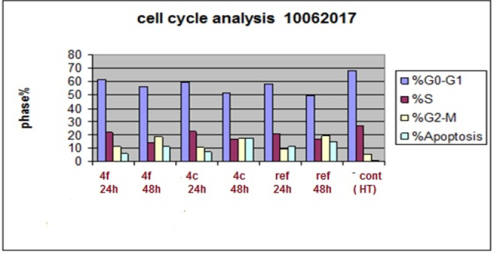
Design, Synthesis, Antitumor activity, cell cycle analysis and ELISA assay for Cyclin Dependant Kinase-2 of a new (4-aryl-6-flouro-4H-benzo[4, 5] thieno[3, 2-b] pyran) derivatives.
Abstract
Full Text:
PDFReferences
J. F. Kerr, A.H. Wyllie, and A.R. Currie, Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British journal of cancer, 1972. 26(4), 239.
G. Häcker, The morphology of apoptosis. Cell and tissue research, 2000. 301(1), 5-17.
F. H. Igney and P.H. Krammer, Death and anti-death: tumour resistance to apoptosis. Nature Reviews Cancer, 2002. 2(4), 277-288.
J. F. Kerr, C.M. Winterford, and B.V. Harmon, Apoptosis. Its significance in cancer and cancer therapy. Cancer, 1994. 73(8), 2013-2026.
N. Morales, et al., Synthesis, Biological Evaluation, and Molecular Simulation of Chalcones and Aurones as Selective MAO-B Inhibitors. Chemical biology & drug design, 2015. 85(6), 685-695.
S. K. Warkhade and K.P. Kakade, Synthesis and characterization of substituted 5 bromo 2-benzylidine-1-benzofuran-3-one and its structural determination.
H. Sedlacek, et al., Flavopiridol (L86 8275; NSC 649890), a new kinase inhibitor for tumor therapy. International journal of oncology, 1996. 9(6), 143-1168.
N. J. Lawrence, et al., The total synthesis of an aurone isolated from Uvaria hamiltonii: aurones and flavones as anticancer agents. Bioorganic & medicinal chemistry letters, 2003. 13(21), 3759-3763.
M. Cushman, D. Nagarathnam, and R.L. Geahlen, Synthesis and evaluation of hydroxylated flavones and related compounds as potential inhibitors of the protein-tyrosine kinase p56lck. Journal of natural products, 1991. 54(5), 1345-1352.
M. T. Konieczny, et al., Synthesis and cytostatic activity of 4, 7-dihydroxythioaurone derivatives. Effect of B ring substitution on the activity. Chemical and pharmaceutical bulletin, 2006. 54(3), 350-353.
B. C. Jennings,et al., 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo [b] thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. Journal of lipid research, 2009. 50(2), 233-242.
I. A. Prior, et al., GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nature Cell Biology, 2001, 3(4), 368-375.
A. F. Zaher, et al., Synthesis, antitumor screening and cell cycle analysis of novel benzothieno [3, 2-b] pyran derivatives. Journal of enzyme inhibition and medicinal chemistry, 2016. 31(sup4), 145-153.
A. E. Hammam, Novel fluoro substituted benzo [b] pyran with anti-lung cancer activity. 2005.
C. E. Dalgliesh and F.G. Mann, 237. The comparative reactivity of the carbonyl groups in the thionaphthenquinones. Part II. The influence of substituent groups in the thionaphthenquinones. Journal of the Chemical Society (Resumed), 1945, 893-909.
M. Bakhouch, et al., Michael addition of active methylene compounds to (Z)-2-arylidenebenzo [b] thiophen-3 (2H)-ones. Mediterranean Journal of Chemistry, 2015. 4(1), 9-17.
M. Bakhouch, et al., Crystal structure of ethyl 2-amino-4-(4-chlorophenyl)-4H-1-benzothieno [3, 2-b] pyran-3-carboxylate. Acta Crystallographica Section E: Crystallographic Communications, 2015. 71(8), o619-o620.
P. Pozarowski, and Z. Darzynkiewicz, Analysis of cell cycle by flow cytometry. Checkpoint Controls and Cancer: Volume 2: Activation and Regulation Protocols, 2004, 301-311.
B. R. Walker, et al., Davidson's principles and practice of medicine. 22nd edition. ed. 2014, Edinburgh ; New York: Churchill Livingstone/Elsevier. xix, 1372 pages.
R. H. Shoemaker, The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer, 2006. 6(10): p. 813-823.
Z. A. Stewart, M.D. Westfall, and J.A. Pietenpol, Cell-cycle dysregulation and anticancer therapy. Trends in Pharmacological Sciences, 2003. 24(3): p. 139-145.
D. Wlodkowic, J. Skommer, and Z. Darzynkiewicz, Flow cytometry-based apoptosis detection. Methods in molecular biology (Clifton, N.J.), 2009. 559: p. 10.1007/978-1-60327-017-5_2.
J. J. Luke, et al., The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: preclinical investigations and phase I clinical trial. Clinical Cancer Research, 2012.
J. Rolff, et al., Preclinical study of a combination of erlotinib and bevacizumab in early stages of unselected non-small cell lung cancer patient-derived xenografts. Targeted oncology, 2016. 11(4): p. 507-514.
D. R. Spigel, et al., Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. 2014, American Society of Clinical Oncology.
DOI: http://dx.doi.org/10.13171/mjc65/01709262240-zaher
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Mediterranean Journal of Chemistry
