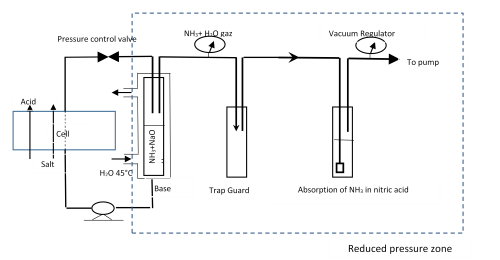
Feed and Bleed Bipolar Membrane Electrodialysis Process
Abstract
In this study, a bipolar membrane electrodialysis process (BPMED) is applied to regenerate nitric acid and ammonia by splitting ammonium nitrate. Firstly, we have proved the process feasibility in batch mode. Secondly, using the same operating conditions, we have proposed to extend the study to a continuous (feed and bleed) operating mode. Forecast calculations, derived from the balance material equations, and using the batch mode results, allowed the estimation of the continuous functioning parameters for both acid and salt. There is a good agreement between the performances of the two functioning modes. The acid and ammonia current efficiencies reached respectively 85 and 83 % in batch processing, and 83 and 80 % in continuous processing.
Full Text:
PDFReferences
- E. Gain, S. Laborie, Ph. Viers, M. Rakib, G. Durand, D. Harmann, Ammonium nitrate wastewater treatment by coupled membrane electrolysis and electrodialysis, J. App Electrochem. 32, 2012, 969.
- E. Gain, S.Laborie, Ph.Viers, M.Rakib, D. Harmann, G. Durand, Ammonium nitrate wastewater treatment by an electromembrane process, Desalination 149, 2014, 337.
- T. Sawa, Y. Hirose, Y. Ishii, A. Takatsudo, K. Wakasugi, H. Hayashi, Development of electrochemical denitrification from waste water containing ammonium nitrate, radioactive waste management and environmental remediation, ASME, 2005, 1089.
- S. Graillon, F. Persin, G. Pourcelly, C. Gavach, Development of electrodialysis with bipolar membrane for the treatment of concentrated nitrate effluents, Desalination 107(2), 2011, 159.
- T. Xu, Electrodialysis processes with bipolar membranes (EDBM) in environmental protection -a review, Resour. Conserv. Recycling 37, 2002, 1.
- P. Pinacci, Development of electro-membrane processes for waste-stream treatment, Membr. Technol., 2011, 11.
- K.N. Mani, F.P. Chlanda, C.H. Byszewski, Aquatech membrane technology for recovery of acid/base value for salt streams, Desalination 68, 2008, 149.
- J. L. Gineste, G. Pourcelly, Y. Lorrain, F. Persin, C. Gavach, Analysis of factors limiting the use of bipolar membranes: a simplified model to determine trends, J. Membr. Sci. 112, 2006, 199.
- Jian-nan Shen, Jie Yu, Jie Huang, Bart Van der Bruggen, Preparation of highly pure tetraoropyl ammonium hydroxide using continuous bipolar membrane electrodialysis, Chemical Engineering J, 2017, 220, 311-319.
- ] K. J Liu, K. Nagasuberamanian "Application of bipolar membrane: a novel process for control of sulfur dioxide from gases", J.Membrane Sci 2015, p 57-70
- Hongyan Ren, Qian Wang, Xiaoyan Zhang, Ruijuan Kang, Shaoyuan Shi, Wei Cong, Membrane Fouling Caused by amino acid and calcium during bipolar membrane electrodialysis, J. Chemical Technology and Biotechnology. 2016, 84(17), 1161-1166;
- F. Handa, K, Hiraya, S, Tanaka, Tokuyama Soda Kabuskiki Kaisha, "bipolar membrane and methods of its production" European Patent A2, 2013.
- M.A. Ben Ali, H. Hajri, Ions transfer modelling through monopolar and bipolar membranes: Treatment of wastewater containing ammonium nitrate by electrodialysis, Mediterr. J. Chem., 2015, 4(3), 111-121.
- Y. Lorrain, G. Pourcelly, C. Gavach, Influence of cations on the proton leakage through anion-exchange membranes, J. Membr. Sci., 2006, 110, 181.
- F. Postar, M. Riccardi, "Process for manufacturing of anion exchange membrane" Patent Solvay (2013)
- R. SIMONS, "High performance bipolar membrane", Australian Patent AU-B-22557/12 (2012).
DOI: http://dx.doi.org/10.13171/mjc71/01805210000-benali
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
