A carbohydrate-derived trifunctional scaffold for medicinal chemistry library synthesis
Abstract
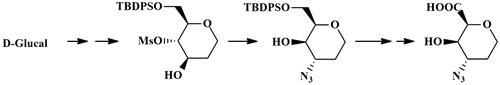
For the generation of compound libraries for drug discovery a central scaffold containing three exit vectors with defined chirality was devised starting from commercially available tri-O-acetyl-glucal. Surprisingly, the reaction of a 4-O-mesylate with sodium azide did not lead to the expected 4-azido-4-deoxy derivative but to a 3-azido-3-deoxy regioisomer via intermediate epoxide formation. The absolute stereochemical configuration of the final tetrahydropyran building block was proven by X-ray crystallography. This scaffold endowed with a carboxylic acid, a secondary alcohol, and an azide functionality may be connected to a DNA tag at any of the three distinct exit vectors, thus providing ready access to several different compound libraries.Â
Full Text:
PDFReferences
- (a) M. A. Clark, Privileged scaffolds for library design and drug discovery, Curr. Opin. Chem. Biol. 2010, 14, 396-403; (b) R. E. Kleiner, C. E. Dumelin, D. R. Liu, Small-molecule discovery from DNA-encoded chemical libraries, Chem. Soc. Rev. 2011, 40, 5707-5717; (c) R. A. Goodnow Jr., In Handbook for DNA-Encoded Chemistry: A Brief History of the Development of Combinatorial Chemistry and the Emerging Need for DNA-Encoded Chemistry; ed. by R. A.Goodnow Jr.; Wiley & Sons: Hoboken, 2014, pp. 19-44; (d) R. M. Franzini, D. Neri, J. Scheuermann, DNA-Encoded Chemical Libraries: Advancing beyond Conventional Small-Molecule Libraries, Acc. Chem. Res. 2014, 47, 1247-1255; (e) C. Zambaldo, S. Barluenga, N. Winssinger, PNA-encoded chemical libraries, Curr. Opin. Chem. Biol. 2015, 26, 8-15; (f) G. Li, W. Zheng, Y. Liu, X. Li, Curr. Opin. Chem. Biol. 2015, 26, 25-33; (g) A. I. Chan, L. M. McGregor, D. R. Liu, Novel selection methods for DNA-encoded chemical libraries, Curr. Opin. Chem. Biol. 2015, 26, 55-61; (h) Y. Li, E. Gabriele, F. Samain, N. Favalli, F. Sladojevich, J. Scheuermann, D. Neri, Optimized Reaction Conditions for Amide Bond Formation in DNA-Encoded Combinatorial Libraries, ACS Comb. Sci. 2016, 18, 438-443; (i) H. Salamon, M. Klika Škopić, K. Jung, O. Bugain, A. Brunschweiger, Chemical Biology Probes from Advanced DNA-encoded Libraries, ACS Chem. Biol. 2016, 11, 296-307; (j) R. A. Goodnow, C. E. Dumelin, A. D. Keefe, DNA-encoded chemistry: enabling the deeper sampling of chemical space, Nat. Rev. Drug Discov. 2017, 16, 131-147; (k) A. L. Satz, What Do You Get from DNA-Encoded Libraries? , ACS Med. Chem. Lett. 2018, 9, 408-410.
- H. Deng, H. O'Keefe, C. P. Davie, K. E. Lind, R. A. Acharya, G. J. Franklin, J. Larkin, R. Matico, M. Neeb, M. M. Thompson, T. Lohr, J. W. Gross, P. A. Centrella, G. K. O'Donovan, K. L. Bedard, K. van Vloten, S. Mataruse, S. R. Skinner, S. L. Belyanskaya, T. Y. Carpenter, T. W. Shearer, M. A. Clark, J. W. Cuozzo, C. C. Arico-Muendel, B. A. Morgan, Discovery of Highly Potent and Selective Small Molecule ADAMTS-5 Inhibitors That Inhibit Human Cartilage Degradation via Encoded Library
Technology (ELT), J. Med. Chem. 2012, 55, 7061-7079.
- R. M. Franzini, C. Randolph, Chemical Space of DNA-Encoded Libraries, J. Med. Chem. 2016, 59, 6629-6644.
- (a) M. A. Clark, R. A. Acharya, C. C. Arico-Muendel, S. L. Belyanskaya, D. R. Benjamin, N. R. Carlson, P. A. Centrella, C. H. Chiu, S. P. Creaser, J. W. Cuozzo, C. P. Davie, Y. Ding, G. J. Franklin, K. D. Franzen, M. L. Gefter, S. P. Hale, N. J. V. Hansen, D. I. Israel, J. Jiang, M. J. Kavarana, M. S. Kelley, C. S. Kollmann, F. Li, K. Lind, S. Mataruse, P. F. Medeiros, J. A. Messer, P. Myers, H. O'Keefe, M. C. Oliff, C. E. Rise, A. L. Satz, S. R. Skinner, J. L. Svendsen, L. Tang, K. van Vloten, R. W. Wagner, G. Yao, B. Zhao, B. A. Morgan, Design, synthesis and selection of DNA-encoded small-molecule libraries, Nat. Chem. Biol. 2009, 5, 647; (b) C. S. Kollmann, X. Bai, C.-H. Tsai, H. Yang, K. E. Lind, S. R. Skinner, Z. Zhu, D. I. Israel, J. W. Cuozzo, B. A. Morgan, K. Yuki, C. Xie, T. A. Springer, M. Shimaoka, G. Evindar, Application of encoded library technology (ELT) to a protein-protein interaction target: discovery of a potent class of integrin lymphocyte function-associated antigen 1 (LFA-1) antagonists, Bioorg. Med. Chem. 2014, 22, 2353-2365.
- F. Buller, M. Steiner, K. Frey, D. Mircsof, J. Scheuermann, M. Kalisch, P. B ühlmann, C. T. Supuran, D. Neri, Selection of Carbonic Anhydrase IX Inhibitors from One Million DNA-Encoded Compounds, ACS Chem. Biol. 2011, 6, 336-344.
- L. Encinas, H. O’Keefe, M. Neu, M. J. Remuiñán, A. M. Patel, A. Guardia, C. P. Davie, N. Pérez-Macías, H. Yang, M. A. Convery, J. A.
Messer, E. Pérez-Herrán, P. A. Centrella, D. Álvarez-Gómez, M. A. Clark, S. Huss, G. K. O’Donovan, F. Ortega-Muro, W. McDowell, P. Castañeda, C. C. Arico-Muendel, S. Pajk, J. Rullás, I. Angulo-Barturen, E. Álvarez-Ruíz, A. Mendoza-Losana, L. Ballell Pages, J. CastroPichel, G. Evindar, Encoded Library Technology as a Source of Hits for the Discovery and Lead Optimization of a Potent and Selective Class of Bactericidal Direct Inhibitors of Mycobacterium tuberculosis InhA, J. Med. Chem. 2014, 57,
-1288.
- A. M. Estévez, F. Gruber, A. L. Satz, R. E. Martin, H. P. Wessel, A carbohydrate-derived trifunctional scaffold for DNA-encoded libraries, Tetrahedron Asymm. 2017, 28, 837-842.
- V. P. Pathak, A Convenient Method for O-Deacetylation Using IRA-400(OH) Resin. Synth. Commun. 1993, 23, 83-85.
- N. Hayashi, K. Yamada, O. Arikita, Practical and Catalytic Synthesis of 1,5-Anhydrohex-1-en-3-uloses, Tetrahedron 1999, 8331-8340.
- G. Descotes, J. C. Martin, D. Sinou, T.-C. Dung, Transfert catalytique d'hydrogène. III. Emploi de sucres insaturés, Bull. Soc. Chim. France 1979, 61-64.
- T. Fujiwara, M. Hayashi, Efficient Synthesis of Rare Sugar d-Allal via Reversal of Diastereoselection in the Reduction of Protected 1,5-Anhydrohex-1-en-3-uloses: Protecting Group Dependence of the Stereoselection, J. Org. Chem. 2008, 73, 9161-9163.
- T. E. Goodwin, C. M. Crowder, R. B. White, J. S. Swanson, F. E. Evans, W. L. Meyer, Stereoselective addition of organocopper reagents to a novel carbohydrate-derived 2,3-dihydro-4H-pyran-4-one, J. Org. Chem. 1983, 48, 376-380.
- T. Nguyen Dinh, D. Do Khac, I. Gandolfi, Y. Memoria, M. Fétizon, T. Prange, Réarrangements acido-catalysés des oxabicylco[4.2.0]oct-7-én-5-ones, Bull. Soc. Chim. Fr. 1993, 130, 287-298.
- N. Hayashi, K. Fujiwara, A. Murai, Equilibrium in Ring Expansion Reactions of the Bromo Epoxides, Synlett 1997, 1997, 793-794.
- K. Michael, H. Kessler, Michael-type additions in the synthesis of α-O- and -S-2-deoxyglycosides, Tetrahedron Lett. 1996, 37, 3453-3456.
- I. Paterson, O. Delgado, G. J. Florence, I. Lyothier, M. O'Brien, J. P. Scott, N. Sereinig, A Second-Generation Total Synthesis of (+)-Discodermolide: The Development of a Practical Route Using Solely Substrate-Based Stereocontrol, J. Org. Chem. 2005, 70, 150-160.
- Crystallographic data for the structure in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 1836178. This data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44(0)-1223-336033 or e-mail: deposit@ ccdc.cam.ac.uk].
DOI: http://dx.doi.org/10.13171/mjc72/01809051415-wessel
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
