An alternative approach to 2-amino-phenylphosphonic acid
Abstract
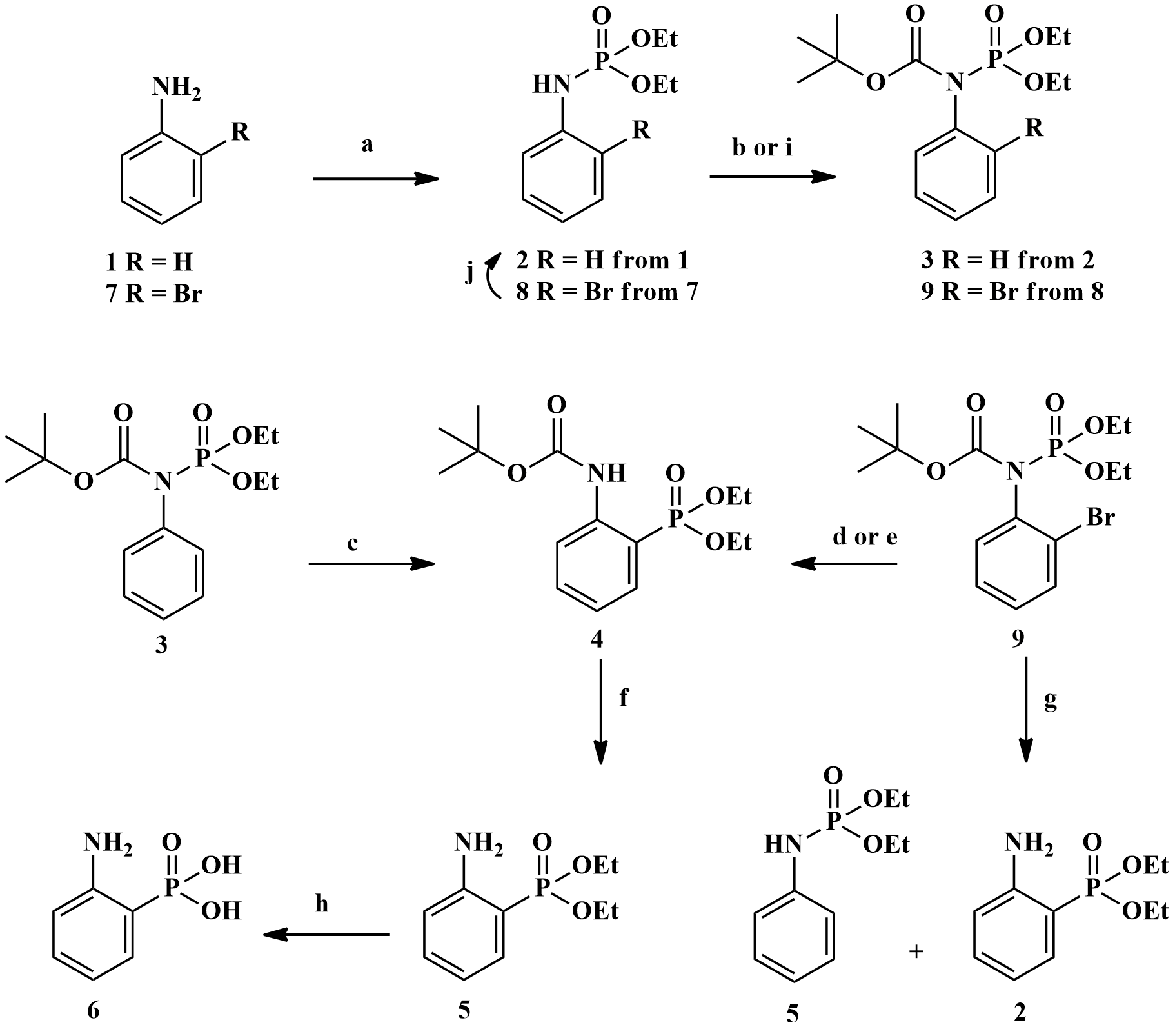
2-Amino-phenlyphosphonic acid can easily be prepared in five steps from aniline in 38% over-all yield with a phosphoramidate-aminophosphonate rearrangement reaction as the key-step.
Â
Full Text:
PDFReferences
- A. Bessmertnykh, C.M. Douaihy, R. Guilard, Direct Synthesis of Amino-substituted Aromatic Phosphonates via Palladium-catalyzed Coupling of Aromatic Mono- and Dibromides with Diethyl Phosphite, Chem. Lett. 2009, 38, 738-739.
T. Hirao, T. Masunaga, Y. Ohshiro, T. Agawa, A Novel Synthesis of Dialkyl Arene-phosphonates, Synthesis, 1981, 56-57.
- T. Hirao, T. Masunaga, N. Yamada, Y. Ohshiro, T. Agawa, Palladium-Catalyzed New Carbon-Phosphorus Bond Formation, B. Chem. Soc. Jpn. 1982, 55, 909-913.
- X.Y. Lu, Z.J. Ni, Palladium-Catalyzed Synthesis of Allylic and Benzylic Sulfides from the Corresponding Dithiocarbonates, Synthesis, 1987, 66-68.
- X.Y. Lu, J.Y. Zhu, Palladium-Catalyzed Reaction of Aryl Polyfluoroalkanesulfonates with O,O-Dialkyl Phosphonates, Synthesis, 1987, 726-727.
- N. Defacqz, B. de Bueger, R. Touillaux, A. Cordi, J. Marchand-Brynaert, Direct phosphonylation of mono- and dihalogenoanilines, Synthesis, 1999, 1368-1372.
- P. Tavs, F. Korte, Zur Herstellung Aromatischer Phosphonsaureester Aus Arylhalogeniden Und Trialkylphosphiten, Tetrahedron, 1967, 23, 4677-4679.
- T.M. Balthazor, R.C. Grabiak, Nickel-Catalyzed Arbuzov Reaction - Mechanistic Observations, J. Org. Chem. 1980, 45, 5425-5426.
- R.C. Grabiak, J.A. Miles, G.M. Schwenzer, Synthesis of Phosphonic Dichlorides and Correlation of Their P-31 Chemical-Shifts, Phosphorus Sulfur 1980, 9, 197-202.
- P. Tavs, Reaction of Aryl Halides with Trialkyl Phosphites and Dialkyl Benzenephosphonites to Aromatic Phosphonates and Phosphinates by Nickel Salt Catalysed Arylation, Chem. Ber. 1970, 103, 2428-2436.
- J.F. Bunnett, E. Mitchel, C. Galli, The Effect of Ortho Substituents in Srn1 Reactions - Some Synthetic Applications, Tetrahedron 1985, 41, 4119-4132.
- J.B. Plumb, R. Obrycki, C.E. Griffin, Phosphonic Acids and Esters. XVI. Formation of Dialkyl Phenylphosphonates by the Photoinitiated Phenylation of Trialkyl Phosphites1,2, The J. Org. Chem. 1966, 31, 2455-2458.
- G. Yang, J. Chun, H. ArakawaUramoto, X. Wang, M.A. Gawinowicz, K. Zhao, D.W. Landry, Anti-cocaine catalytic antibodies: A synthetic approach to improved antibody diversity, J. Am. Chem. Soc. 1996, 118, 5881-5890.
- Z.E. Golubski, Z. Skrowaczewska, New Synthesis of Cyclic Esters of Phosphonic and Thiophosphonic Acids, Synthesis, 1979, 21-23.
- S. Yasui, M. Fujii, C. Kawano, Y. Nishimura, K. Shioji, A. Ohno, Mechanism of Dediazoniation of Arenediazonium Salts with Triphenylphosphine and Trialkyl Phosphites - Generation of Cation Radicals from Trivalent Phosphorus-Compounds and Their Reactions, J. Chem. Soc. Perkin Trans. 2, 1994, 177-183.
- F. Effenberger, H. Kottmann, Oxidative Phosphonylation of Aromatic-Compounds, Tetrahedron 1985, 41, 4171-4182.
- Y.M. Kargin, E.V. Nikitin, O.V. Parakin, G.V. Romanov, A.N. Pudovik, Electrochemical Synthesis of Organo-Phosphorus Compounds, Phosphorus Sulfur 1980, 8, 55-58.
- H. Kottmann, J. Skarzewski, F. Effenberger, Oxidative Phosphonylation of Aromatics with Ammonium Cerium(Iv) Nitrate, Synthesis 1987, 797-801.
- A.M. Jardine, S.M. Vather, T.A. Modro, Metalation-Induced Migration of Phosphorus from Nitrogen to Carbon, J. Org. Chem. 1988, 53, 3983-3985.
- S. Masson, J.F. SaintClair, A. Dore, M. Saquet, Phosphorothioate-mercaptophosphonate rearrangement: Synthesis of new o-mercaptoaryl- and o-mercaptoheteroaryl phosphonates and their derivatives, Bull. Soc. Chim. Fr. 1996, 133, 951-964.
- S.S. De Silva, P.J. Camp, D.K. Henderson, D.C.R. Henry, H. McNab, P.A. Tasker, P. Wight, Attachment of phosphonate-functionalised azo-dyes to oxide surfaces to give enhanced light and wet fastness, Chem. Commun. 2003, 1702-1703.
- A. Mucha, A. Kunert, J. Grembecka, M. Pawelczak, P. Kafarski, A phosphonamidate containing aromatic N-terminal amino group as inhibitor of leucine aminopeptidase - design, synthesis and stability, Eur. J. Med. Chem. 2006, 41, 768-772.
- Y.C. Kim, S.G. Brown, T.K. Harden, J.L. Boyer, G. Dubyak, B.F. King, G. Burnstock, K.A. Jacobson, Structure-activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X(1) receptors, J. Med. Chem. 2001, 44, 340-349.
- R. Beugelmans, M. Chbani, Photostimulated S(Rn)1 Reactions on Functionalized Aryl Bromides Enhanced by Kl Addition - Synthetic and Mechanistic Aspects, New J. Chem. 1994, 18, 949-952.
- K. Issleib, R. Vollmer, o-Substituted benzenephosphonic acid diethyl ester and o-amino, o-hydroxy, and o-mercaptophenyl phosphine, Z. Chem. 1978, 18, 451-452.
- F. Hammerschmidt, E. Schneyder, E. Zbiral, Novel synthetic aspects of the phosphonate-phosphate rearrangement. 1. A useful approach to 1,2-propadienyl phosphates, Chem. Ber. 1980, 113, 3891-3897.
- F. Hammerschmidt, E. Zbiral, Novel synthetic aspects of the phosphonate-phosphate-rearrangement. II. Synthesis of enolphosphates from 1-oxoalkanphosphonates and sulfur ylides, Monatsh. Chem. 1980, 111, 1015-1023.
- F. Hammerschmidt, S. Schmidt, The phosphonate-phosphate and phosphate-phosphonate rearrangement and their applications. Part 4. Deprotonation of secondary benzylic phosphates. Configurationally stable benzylic carbanions with a diethoxyphosphoryloxy substituent and their rearrangement to optically active tertiary α-hydroxy phosphonates, Chem. Ber. 1996, 129, 1503-1508.
- F. Hammerschmidt, S. Schmidt, The phosphonate-phosphate and phosphate-phosphonate rearrangement and their applications. Part 5. On the reaction of sec-butyllithium/TMEDA with symmetrical trialkyl phosphates, Monatsh. Chem. 1997, 128, 1173-1180.
- F. Hammerschmidt, H. Voellenkle, Stereochemistry of the phosphate-phosphonate rearrangement, Liebigs Ann. Chem. 1986, 2053-2064.
- N.V. Kolotilo, A.A. Sinitsa, Y.V. Rassukanaya, P.P. Onys'ko, N-sulfonyl-and N-phosphoryl-benzimidoylphosphonates, Russ. J. Gen. Chem. 2006, 76, 1210-1218.
- A.N. Pudovik, M.G. Zimin, I.V. Konovalova, V.M. Pozhidaev, L.I. Vinogradov, Aminophosphonate-amidophosphate rearrangement of bis(dialkylphosphono)alkylamines, Zh. Obshch. Khim. 1975, 45, 30-37.
- B. Dhawan, D. Redmore, Rearrangement of a di-tert-butyl aryl phosphate to a di-tert-butyl (2-hydroxyaryl)phosphonate. A convenient preparation of (2-hydroxyphenyl)- and (2-hydroxy-5-methoxyphenyl)phosphonic acids, Synth. Commun. 1985, 15, 411-416.
- B. Dhawan, D. Redmore, Rearrangement of di-tert-butyl aryl phosphates to di-tert-butyl (2-hydroxyaryl)phosphonates. Preparation of (2-hydroxy-1,3-phenylene)bisphosphonic acids, Phosphorus, Sulfur Silicon Relat. Elem. 1989, 42, 177-182.
- B. Dhawan, D. Redmore, Metalation-induced 1,3-migration of a diphenylphosphinyl group from oxygen to carbon. Preparation of 2-(diphenylphosphinyl)phenols. J. Chem. Res., Synop.1989, 328.
- E. Kuliszewska, F. Hammerschmidt, On the rearrangement of N-aryl-N-Boc-phosphor-
amidates to N-Boc-protected o-aminoaryl-phosphonates, Monatsh. Chem. 2018, 149, 87-98.
- J.-L. Paparin, A. Amador, E. Badaroux, S. Bot, C. Caillet, T. Convard, D. Da Costa, D. Dukhan, L. Griffe, J.-F. Griffon, M. La Colla, F. Leroy, M. Liuzzi, A.G. Loi, J. McCarville, V. Mascia, J. Milhau, L. Onidi, C. Pierra, R. Rahali, E. Rosinosky, E. Sais, M. Seifer, D. Surleraux, D. Standring, C.B. Dousson, Discovery of benzophosphadiazine drug candidate IDX375: A novel hepatitis C allosteric NS5B RdRp inhibitor, Bioorg. Med. Chem. Lett. 2017, 27, 2634-2640.
- Y. Wang, J. Desai, Y. Zhang, S.R. Malwal, C.J. Shin, X. Feng, H. Sun, G. Liu, R.-T. Guo, E. Oldfield, Bacterial Cell Growth Inhibitors Targeting Undecaprenyl Diphosphate Synthase and Undecaprenyl Diphosphate Phosphatase, ChemMedChem. 2016, 11, 2311-2319.
- W. Dabkowski, J. Michalski, C. Radziejewski, Z. Skrzypczynski, Phosphoric and phosphinic sulfonic anhydrides - reinvestigation and corrections. Novel methods of synthesis, Chem. Ber. 1982, 115, 1636-1643.
- G.O. Doak, L.D. Freedman, Synthesis of phosphanilic acid and related compounds, J. Am. Chem. Soc. 1952, 74, 753-754.
DOI: http://dx.doi.org/10.13171/mjc731892812-csuk
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
