Synthesis, characterization and in vitro biological screening of 4-hydroxy naphthalen-1-yl, naphtho[1,2-b]furan, benzo[h]chromene and 5,6-dihydropyridazine derivatives containing sulfonamide moiety
Abstract
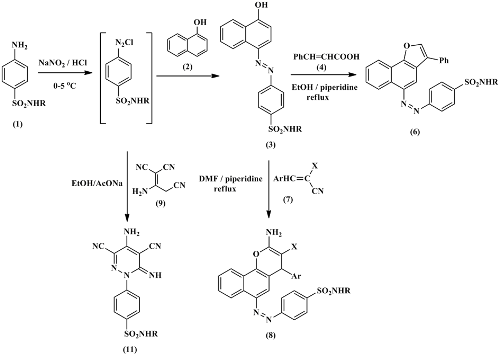
In this study, a series of 4-((4-hydroxynaphthalen-1-yl)diazenyl)benzenesulfonamides have been prepared by subsequent diazotization of sulfonamide derivatives and coupling with 1-naphthol in alkaline medium. Cyclization of 4-((4-hydroxynaphthalen-1-yl)diazenyl)benzenesulfonamides with cinnamic acid in the presence of a basic catalyst afforded the novel naphtho[1,2-b]furans. Also, 4-((4-hydroxynaphthalen-1-yl)diazenyl)benzenesulfonamides can be cyclized with α‐cyanocinnamonitriles to afford 2-amino-3-cyano-4-phenyl-4H-benzo[h]chromenes. 4-(4-amino-3,5- dicyano-6-iminopyridazin-1(6H)-yl)benzenesulfonamides were obtained at room temperature by treatment of 2-amino-1,1,3-tricyanopropene with a diazonium salt of sulfonamide derivatives. The structures of newly synthesized compounds were confirmed by analytical data and spectroscopic techniques. The antimicrobial activity of the obtained compounds was assessed in vitro by qualitative and quantitative (minimum inhibitory concentration) (MIC) assays.
Full Text:
PDFReferences
- S. Omidi, V. Khojasteh, A. Kakanejadifard, M. Ghasemian, and F. Azarbani, Synthesis, characterization, spectroscopy and biological activity of 4-((3-formyl-4-hydroxyphenyl) azo)-1-alkylpyridinium salts, J. Chem. Sci., 2018, 130, 114.
- R. Rahman, A. Gul, and Z. Akhter, 4, 4′-Bis
-(4′-hydroxyphenyl) phenylazo] diphenyl Ether, Molbank, 2016, 2016, M914.
- E. Merino, Synthesis of azobenzenes: the coloured pieces of molecular materials, Chem. Soc. Rev., 2011, 40, 3835-3853.
- H. Zollinger, Color Chemistry-Synthesis, Properties and Application of Organic Dyes and Pigments, VCH Publ. New York, 1987, 85-87.
- P. F. Gordon, Organic Chemistry in Color, Springer: New York, NY, USA, 1987, 95-105.
- D. Stevenson, Tartrazine, Azo, and Non-Azo Dyes. Food Allergy, 2008, 377.
- X. Cheng, Q. Li, C. Li, J. Qin, and Z. Li, Azobenzeneâ€Based Colorimetric Chemosensors for Rapid Nakedâ€Eye Detection of Mercury (II), Chem. Eur. J., 2011, 17, 7276-7281.
- W. J. Sandborn, Rational selection of oral 5-aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. Nature Publishing Group, 2002.
- K. Morihiro, O. Hasegawa, S. Mori, S. Tsunoda, and S. Obika, C5-azobenzene-functionalized locked nucleic acid uridine: isomerization properties, hybridization ability, and enzymatic stability, Org. Biomol. Chem., 2015, 13, 5209-5214.
- L. Fang, S. Chen, X. Guo, Y. Zhang, and H. Zhang, Azobenzene-containing molecularly imprinted polymer microspheres with photo-and thermoresponsive template binding properties in pure aqueous media by atom transfer radical polymerization, Langmuir, 2012, 28, 9767-9777.
- Y. Wang et al., Block copolymer aggregates with photo-responsive switches: towards a controllable supramolecular container, Polymer (Guildf)., 2009, 50, 4821-4828.
- A. Natansohn and P. Rochon, Photoinduced motions in azo-containing polymers, Chem. Rev., 2002, 102, 4139-4176.
- K. Itoga, M. Yamato, J. Kobayashi, A. Kikuchi, and T. Okano, Cell micropatterning using photopolymerization with a liquid crystal device commercial projector, Biomaterials, 2004, 25, 2047-2053.
- T. Jaunet-Lahary, A. Chantzis, K. J. Chen, A. D. Laurent, and D. Jacquemin, Designing efficient azobenzene and azothiophene nonlinear optical photochromes, J. Phys. Chem. C, 2014, 118, 28831-28841.
- W. R. Browne and B. L. Feringa, Making molecular machines work, Nat. Nanotechnol., 2006, 1, 25-35.
- T. Lin et al., Red, green and blue reflections enabled in an optically tunable selfâ€organized 3D cubic nanostructured thin film, Adv. Mater., 2013, 25, 5050-5054.
- C. H. Lee, B. Bihari, R. Filler, and B. K. Mandal, New azobenzene non-linear optical materials for eye and sensor protection, Opt. Mater. (Amst)., 2009, 32, 147-153.
- O. Graydon, Photoisomerization: Molecular motors driven by light, Nat. Photonics, 2015, 9, 13.
- L. Xia, A. Idhayadhulla, Y. R. Lee, Y.-J. Wee, and S. H. Kim, Anti-tyrosinase, antioxidant, and antibacterial activities of novel 5-hydroxy-4-acetyl-2, 3-dihydronaphtho [1, 2-b] furans, Eur. J. Med. Chem., 2014, 86, 605-612.
- L. Xia and Y. R. Lee, Regioselective synthesis of novel and diverse naphtho [1, 2-b] furan-3-carboxamides and benzofuran-3-carboxamides by cascade formal [3+ 2] cycloaddition, RSC Adv., 2014, 4, 36905-36916.
- C.-Y. Hsieh, P.-C. Tsai, C.-H. Tseng, Y. Chen, L.-S. Chang, and S.-R. Lin, Inhibition of EGF/EGFR activation with naphtho [1, 2-b] furan-4, 5-dione blocks migration and invasion of MDA-MB-231 cells, Toxicol. Vitr., 2013, 27, 1-10.
- Y. Chen et al., Discovery of N-(Naphtho [1, 2-b] Furan-5-Yl) Benzenesulfonamides as Novel Selective Inhibitors of Triple-Negative Breast Cancer (TNBC), Molecules, 2018, 23, 678.
- S. A. Patil, R. Patil, L. M. Pfeffer, and D. D. Miller, Chromenes: potential new chemotherapeutic agents for cancer, Future Med. Chem., 2013, 5, 1647-1660.
- Y. Dong, K. Nakagawa-Goto, C.-Y. Lai, S. L. Morris-Natschke, K. F. Bastow, and K.-H. Lee, Antitumor agents 278. 4-Amino-2H-benzo [h] chromen-2-one (ABO) analogs as potent in vitro anti-cancer agents, Bioorg. Med. Chem. Lett., 2010, 20, 4085-4087.
- Y. Dong, K. Nakagawa-Goto, C.-Y. Lai, S. L. Morris-Natschke, K. F. Bastow, and K.-H. Lee, Antitumor agents 281. Design, synthesis, and biological activity of substituted 4-amino-7, 8, 9, 10-tetrahydro-2H-benzo [h] chromen-2-one analogs (ATBO) as potent in vitro anticancer agents, Bioorg. Med. Chem. Lett., 2011, 21, 546-549.
- Y. Dong et al., Antitumor agents. 272. Structure-Activity relationships and in vivo selective anti-breast cancer activity of novel neo-tanshinlactone analogues, J. Med. Chem., 2010, 53, 2299-2308.
- C. T. Supuran and A. Scozzafava, Carbonic anhydrase inhibitors, Curr. Med. Chem. Endocr. Metab. Agents, 2001, 1, 61-97.
- S. M. Monti, C. T. Supuran, and G. De Simone, Anticancer carbonic anhydrase inhibitors: a patent review (2008-2013), Expert Opin. Ther. Pat., 2013, 23, 737-749.
- M. Kaya, E. Demir, and H. Bekci, Synthesis, characterization and antimicrobial activity of novel xanthene sulfonamide and carboxamide derivatives, J. Enzyme Inhib. Med. Chem., 2013, 28, 885-893.
- A. Scozzafava, C. T. Supuran, and F. Carta, Antiobesity carbonic anhydrase inhibitors: literature and patent review, Expert Opin. Ther. Pat., 2013, 23, 725-735.
- B. Żołnowska, J. Sławiński, A. Pogorzelska, J. Chojnacki, D. Vullo, and C. T. Supuran, Carbonic anhydrase inhibitors. Synthesis, and
molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl) guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associa, Eur. J.
Med. Chem., 2014, 71, 135–147.
- S. Bag et al., Sulfonamides as multifunctional
agents for Alzheimer’s disease, Bioorg. Med. Chem. Lett., 2015, 25, 626–630.
- M. S. A. El-Gaby, M. M. Ghorab, Z. H. Ismail, S. M. Abdel-Gawad, and H. M. Aly, Synthesis, structural characterization and anticancer evaluation of pyrazole derivatives, Med. Chem. Res., 2018, 27, 72-79.
- M. M. Ghorab, M. S. Alsaid, M. S. A. El-Gaby, N. A. Safwat, M. M. Elaasser, and A. M. Soliman, Biological evaluation of some new N-(2, 6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents, Eur. J. Med. Chem., 2016, 124, 299-310.
- M. M Ghorab et al., Novel thiourea derivatives bearing sulfonamide moiety as anticancer agents through COX-2 inhibition, Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chem. Agents), 2017, 17, 1411-1425.
- M. M. Ghorab, M. S. A. El-Gaby, A. M. Soliman, M. S. Alsaid, M. M. Abdel-Aziz, and M. M. Elaasser, Synthesis, docking study and biological evaluation of some new thiourea derivatives bearing benzenesulfonamide moiety, Chem. Cent. J., 2017, 11, 42.
- M. M. Ghorab, M. S. Alsaid, M. S. A. El-Gaby, M. M. Elaasser, and Y. M. Nissan, Antimicrobial and anticancer activity of some novel fluorinated thiourea derivatives carrying sulfonamide moieties: synthesis, biological evaluation and molecular docking, Chem. Cent. J., 2017, 11, 32.
- A. M. El-Agrody, A.-A. M. Al-Dies, and A. M. Fouda, Microwave-assisted synthesis of 2-amino-6-methoxy-4H-benzo [h] chromene derivatives, Eur. J. Chem., 2014, 5, 133-137.
- R.-A. Tucaliuc, V. V Cotea, M. Niculaua, C. Tuchilus, D. Mantu, and I. I. Mangalagiu, New pyridazine–fluorine derivatives: Synthesis, chemistry and biological activity. Part II, Eur. J. Med. Chem., 2013, 67, 367-372.
- H.-A. S. Abbas, S. S. A. El-Karim, E. M. Ahmed, A. F. Eweas, and S. A. El-Awdan, Synthesis, biological evaluation and molecular docking studies of aromatic sulfonamide derivatives as anti-inflammatory and analgesic agents, Acta Pol. Pharm., 2016, 73, 1163-1180.
- M. Gupta, R. Gupta, and M. Anand, Hydroxyapatite supported caesium carbonate as a new recyclable solid base catalyst for the Knoevenagel condensation in water, Beilstein J. Org. Chem., 2009, 5, 1-7.
- M. Mittelbach, An improved and facile synthesis of 2-amino-1, 1, 3-tricyanopropene, Monatshefte für Chemie/Chemical Mon., 1985, 116, 689-691.
- E. Committee, S. Testing, C. Microbiology, and I. D. Escmid, Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution, Clin. Microbiol. Infect., 2000, 6, 509-515.
- NCCLS, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically M7-A5. 5th ed. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2000. Wayne (PA): The Committee, 2000.
- M. A. Abdelrahman, I. Salama, M. S. Gomaa, M. M. Elaasser, M. M. Abdel-Aziz, and D. H. Soliman, Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents, Eur. J. Med. Chem., 2017, 138, 698-714.
DOI: http://dx.doi.org/10.13171/mjc751912061355msaeg
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
