Hydroxylated boswellic and glycyrrhetinic acid derivatives: synthesis and cytotoxicity
Abstract
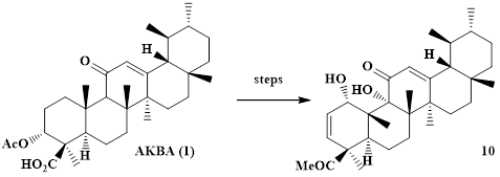
Oxidation of 2,3-dehydro-11-keto--boswellic acid gave derivatives holding extra hydroxyl groups at positions C-1, C-2 or C-1 and C-9, respectively. The synthesis of 2,3-dehydro-1,9-dihydroxy-11-keto--boswellic acid represents the first partial-synthetic access to this class of compounds. The synthetic strategy can be expanded easily, and a corresponding analogue derived from glycyrrhetinic acid was accessed by the same synthetic scheme in good overall yield. Boswellic and glycyrrhetinic acid 1,9-endoperoxides are intermediates for the synthesis of the 1,9-dihydroxylated compounds. These 1,9-endo-peroxides were highly cytotoxic for several human tumor cell lines but only diminished cytotoxicity was observed in SRB assays for the 1,9-dihydroxylated compounds.
Full Text:
PDFReferences
- M. Abdel-Tawab, O. Werz, M. Schubert-Zsilavecz, Boswellia serrata; an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data, Clin. Pharmacokinet. 2011, 50, 349-369.
- Z. Du, Z. Liu, Z. Ning, Y. Liu, Z. Song, C. Wang, A. Lu, Prospects of Boswellic Acids as Potential Pharmaceutics, Planta Med. 2015, 81, 259-271.
- T. Morikawa, H. Matsuda, M. Yoshikawa, A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh, J. Oleo Sci. 2017, 66, 805-814.
- A. Moussaieff, R. Mechoulam, Boswellia resin: from religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials, J. Pharm. Pharmacol. 2009, 61, 1281-1293.
- A. Sharma, S. Chhikara, S. N. Ghodekar, S. Bhatia, M. D. Kharya, V. Gajbhiye, A. S. Mann, A. G. Namdeo, K. R. Mahadik, Phytochemical and pharmacological investigations on Boswellia serrata, Pharmacogn. Rev. 2009, 3, 195-204.
- A. Al-Harrasi, H. Hussain, R. Csuk, Y. K. Khan, Chemistry and bioactivity of boswellic acids and other terpenoids of the genus boswellia; Elsevier, 2018.
- H. P. T. Ammon, Boswellic Acids and Their Role in Chronic Inflammatory Diseases, Adv. Exp. Med. Biol. 2016, 928, 291-327.
- H. Hussain, A. Al-Harrasi, R. Csuk, U. Shamraiz, I. R. Green, I. Ahmed, I. A. Khan, Z. Ali, Therapeutic potential of boswellic acids: a patent review (1990-2015), Expert Opin. Ther. Pat. 2017, 27, 81-90.
- M. A. Khan, R. Ali, R. Parveen, A. K. Najmi, S. Ahmad, Pharmacological evidences for cytotoxic and antitumor properties of Boswellic acids from Boswellia serrata, J. Ethnopharmacol. 2016, 191, 315-323.
- R. Paduch, M. Kandefer-Szerszen, Antitumor and Antiviral Activity of Pentacyclic Triterpenes, Mini-Rev. Org. Chem. 2014, 11, 262-268.
- H. P. T. Ammon, Boswellic acids in chronic inflammatory diseases, Planta Med. 2006, 72, 1100-1116.
- S. C. Ng, Y. T. Lam, K. K. F. Tsoi, F. KL. L. Chan, J. J. Y. Sung, J. C. Y. Wu, Systematic review: the efficacy of herbal therapy in inflammatory bowel disease, Aliment. Pharmacol. Ther. 2013, 38, 854-863.
- C. Anthoni, M. G. Laukoetter, E. Rijcken, T. Vowinkel, R. Mennigen, S. Müller, N. Senninger, J. Russell, J. Jauch, J. Bergmann, D. N. Granger, C. F. Krieglstein, Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis, Am. J. Physiol. 2006, 290, G1131-G1137.
- P. R. Kiela, A. J. Midura, N. Kuscuoglu, S. D. Jolad, A. M. Solyom, D. G. Besselsen, B. N. Timmermann, F. K. Ghishan, Effects of boswellia serrata in mouse models of chemically induced colitis, Am. J. Physiol. 2005, 288, G798-G808.
- A. Sarkate, S. S. DhaneshwarInvestigation of mitigating effect of colon-specific prodrugs of boswellic acid on 2,4,6-trinitrobenzene sulfonic acid-induced colitis in Wistar rats: Design, kinetics and biological evaluation, World J. Gastroenterol. 2017, 23, 1147-1163.
- S. S. Sonje, D. N. Raut, S. R. Chaudhari, M. J. Chavan, Effect of Boswellia serrata Extract Microcapsule Against Ulcerative Colitis in Mice, Nat. Prod. J. 2016, 6, 305-312.
- U. Siemoneit, B. Hofmann, N. Kather, T. Lamkemeyer, J. Madlung, L. Franke, G. Schneider, J. Jauch, D. Poeckel, O. Werz, Identification and functional analysis of cyclooxygenase-1 as a molecular target of boswellic acids, Biochem. Pharmacol. 2008, 75, 503-513.
- U. Siemoneit, C. Pergola, B. Jazzar, H. Northoff, C. Skarke, J. Jauch, O. Werz, On the interference of boswellic acids with 5-lipoxygenase: Mechanistic studies in vitro and pharmacological relevance, Eur. J. Pharmacol. 2009, 606, 246-254.
- U. Siemoneit, L. Tausch, D. Poeckel, M. Paul, H. Northoff, A. Koeberle, J. Jauch, O. Werz, Defined Structure-Activity Relationships of Boswellic Acids Determine Modulation of Ca2+ Mobilization and Aggregation of Human Platelets by Boswellia serrata Extracts, Planta Med. 2017, 83, 1020-1027.
- R. Csuk, A. Barthel-Niesen, A. Barthel, R. Schäfer, A. Al-Harrasi, 11-Keto-boswellic acid derived amides and monodesmosidic saponins induce apoptosis in breast and cervical cancers cells, Eur. J. Med. Chem. 2015, 100, 98-105.
- R. Csuk, A. Niesen-Barthel, A. Barthel, R. Kluge, D. Ströhl, D. Synthesis of an antitumor active endoperoxide from 11-keto-β-boswellic acid, Eur. J. Med. Chem. 2010, 45, 3840-3843.
- R. Csuk, A. Niesen-Barthel, R. Schäfer, A. Barthel, A. Al-Harrasi, Synthesis and antitumor activity of ring A modified 11-keto-β-boswellic acid derivatives, Eur. J. Med. Chem. 2015, 92, 700-711.
- P. Fan, T. Li, Y. Ye, Q. Luo, H. Yuan, H. Lou, Synthesis and cytotoxic activity of boswellic acid analogues, Phytochem. Lett. 2016, 18, 99-104.
- A. Kumar, A. Qayum, P. R. Sharma, S. K. Singh, B. A. Shah, Synthesis of β-boswellic acid derivatives as cytotoxic and apoptotic agents, Bioorg. Med. Chem. Lett. 2016, 26, 76-81.
- T. Li, P. Fan, Y. Ye, Q. Luo, H. Lou, Ring A-modified Derivatives from the Natural Triterpene 3-O-acetyl-11-keto-β-Boswellic Acid and their Cytotoxic Activity, Anti-Cancer Agents Med. Chem. 2017, 17, 1153-1167.
- K. Li, L. Li, S. Wang, X. Li, T. Ma, D. Liu, Y. Jing, L. Zhao, Design and synthesis of novel 2-substituted 11-keto-boswellic acid heterocyclic derivatives as anti-prostate cancer agents with Pin1 inhibition ability, Eur. J. Med. Chem. 2017, 126, 910-919.
- H. Schneider, M. Weller, M. Boswellic acid activity against glioblastoma stem-like cells, Oncol. Lett. 2016, 11, 4187-4192.
- R. K. Wolfram, L. Fischer, R. Kluge, D. Ströhl, A. Al-Harrasi, R. Csuk, Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans, Eur. J. Med. Chem. 2018, 155, 869-879.
- H. Huang, A. Li, F. Zhao, X. Xie, K. Li, Y. Jing, D. Liu, L. Zhao, Design, synthesis and biological evaluation of ring A modified 11-keto-boswellic acid derivatives as Pin 1 inhibitors with remarkable anti-prostate cancer activity, Bioorg. Med. Chem. Lee. 2018, 28, 3187-3193.
- B. Meka, S. R. Ravada, M. K. K. Muthyala, K. P. Nagasree, T. Golakoti, Synthesis of new analogs of AKBA and evaluation of their anti-inflammatory activity, Bioorg. Med. Chem. 2017, 25, 1374-1388.
- S. Shen, X. Xu, Z. Liu, J. Liu, L. Hu, Synthesis and structure-activity relationships of boswellic acid derivatives as potent VEGFR-2 inhibitors, Bioorg. Med. Chem. 1993, 23, 1982-1993.
- A. Kumar, B. A. Shah, S. Singh, A. Hamid, S. K. Singh, V. K. Sethi, A. K. Saxena, J. Singh, S. C. Taneja, Acyl derivatives of boswellic acids as inhibitors of NF-ï«B and STATs, Bioorg. Med. Chem. Lett. 2012, 22, 431-435
- B. Mahajan, S. C. Taneja, V. K. Sethi, K. L. Dhar, Two triterpenoids from Boswellia serrata gum resin, Phytochemistry 1995, 39, 453-455.
- S. Schweizer, A. F. W. Von Brocke, S: E. Boden, E. Bayer, H. P. T. Ammon, H. Safayhi, Workup-Dependent Formation of 5-Lipoxygenase Inhibitory Boswellic Acid Analogues, J. Nat. Prod. 2000, 63, 1058-1061.
- A. Al-Harrasi, N. U. Rehman, A. L. Khan, M. Al-Broumi, I. Al-Amri, H. Hussain, J. Hussain, R. Csuk, Chemical, molecular and structural studies of Boswellia species: β-Boswellic Aldehyde and 3-epi-11β-Dihydroxy BA as precursors in biosynthesis of boswellic acids, PLoS One 2018, 13, e0198666.
- J. Jauch, J. Bergmann, Chemical of boswellic acids, 1. An efficient method for the large-scale preparation of 3-O-acetyl-11-oxo-β-boswellic acid and other boswellic acids, Eur. J. Org. Chem. 2003, 4752-4756.
- R. Csuk, A. Barthel-Niesen, D. Ströhl, R. Kluge, C. Wagner, A. Al-Harrasi, Oxidative and reductive transformations of 11-keto-β-boswellic acid, Tetrahedron 2015, 71, 2025-2034.
- V. Kulcitki, N. Ungur, M. Gavagnin, M. Carbone, G. Cimino, Further synthetic studies towards the austrodorane skeleton: Synthesis of austrodoral, Eur. J. Org. Chem. 2005, 1816-1822.
- J. Baeten, K. Deforce, S. Challe, D. Vos, P. de Degryse, Holy smoke in medieval funerary rites: chemical fingerprints of frankincense in Southern Belgian incense burners, PLoS One 2014, 9, e113142/1-e113142/18, 18 pp.
- O. Werz, J.-F. Kapp, R. Martin, Use of boswellic acids and synthetic boswellic acid derivatives for inhibiting microsomal prostaglandin E2 synthase and cathepsin G, WO2009117987A3.
- O. Werz, U. Siemoneit, A. Henkel, J. Jauch, N. Kather, Use of boswellic acids and synthetic boswellic acid derivatives for inhibiting microsomal prostaglandin E2 synthase and cathepsin G, DE102008015607A1.
- J. Jauch, Simple method for the synthesis of boswellic acids and derivatives thereof, WO2002085921A2.
- A. Chung, M. R. Miner, K. J. Richert, C. J. Rieder, K. A. Woerpel, Formation of an Endoperoxide upon Chromium-Catalyzed Allylic Oxidation of a Triterpene by Oxygen, J. Org. Chem. 2015, 80, 266-273.
- A. Niesen, A. Barthel, R. Kluge, A. Köwitsch, D. Ströhl, S. Schwarz, R. Csuk, Antitumoractive Endoperoxides from Triterpenes, Arch. Pharm. 2009, 342, 569-576.
- S. Schwarz, R. Csuk, R. Synthesis and antitumour activity of glycyrrhetinic acid derivatives, Bioorg. Med. Chem. 2010, 18, 7458-7474.
- S. He, W. Yang, L. Zhu, G. Du, C.-S. Lee, Bioinspired Total Synthesis of (±)-Yezo'otogirin C, Org. Lett. 2014, 16, 496-499.
- Y. Kara, M. Balci, A new and stereospecific synthesis of an inositol analogue: bis-homoinositol, Tetrahedron 2003, 59, 2063-2066.
- M. S. Alam, G. Kaur, A. Ali, H. Hamid, M. Ali, M. Athar, Two new bioactive oleanane triterpene glycosides from Terminalia arjuna, Nat. Prod. Res. 2008, 22, 1279-1288.
- A. Ali, M. Ali, M. S. Alam, Two new oleanane triterpene glycosides from the bark of Terminalia arjuna, Z. Naturforsch., B: Chem. Sci. 2006, 61, 1282-1286.
- R. A. Kaskoos, M. Ali, K. J. Naquvi, New homosesquiterpenol and stigmasteryl digalactoside from the stem bark of Terminalia arjuna, Int. Res. J. Pharm. 2012, 3, 137-139.
- R. A. Kaskoos, M. Ali, K. J. Naquvi, New oleanene triterpenoids from the stem bark of Terminalia arjuna, J. Pharm. Res. 2012, 5, 2368-2372.
- P. Skehan, R, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, M. R. Boyd, New colorimetric cytotoxicity assay for anticancer-drug screening, J. Natl. Cancer Inst. 1990, 82, 1107-1112.
DOI: http://dx.doi.org/10.13171/mjc74181121-csuk
Refbacks
- There are currently no refbacks.
Copyright (c) 2018 Mediterranean Journal of Chemistry
