Diastereoselective Synthesis of some pyrrolidin-2-ones azasugars and study of their stereochemistry
Abstract
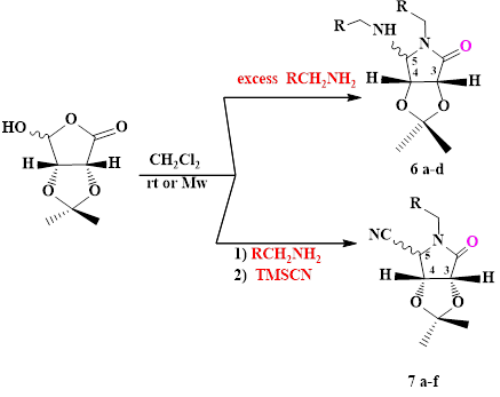
The microwave-promoted one-pot multicomponent synthesis of substituted pyrrolidinols using a bifunctional sugar-derived hydroxy-[gamma]-lactone component of amine component or cyanide group is reported. Clean reaction profile, easy work-up procedure, excellent yields and short reaction times are some remarkable features of this method.
This modular approach features the in situ-generation of iminium ions intermediate that allows the diastereoselective assembly of the diverse pyrrolidinones efficiently under microwave irradiation has been used.
Full Text:
PDFReferences
- T. J. J. M üller. General Discussion and Reactions Involving a Carbonyl Compound as Electrophilic Component. In: T. J. J. M üller., editor. Multicomponent Reactions 1. Stuttgart: Georg Thieme Verlag KG, 2014. pp. 5-27. (Science of Synthesis Series).
- D.M. D'Souza, T. J.J. M üller, Multi-component syntheses of heterocycles by transition-metal catalysis Chem. Soc. Rev, 2007, 36, 1095-1108.
- H. Ahankar, A. Ramazani, K.Slepokura, T. Lis, S-W. Joo, Synthesis of pyrrolidinone derivatives from aniline, an aldehyde and diethyl acetylenedicarboxylate in an ethanolic citric acid solution under ultrasound irradiation, Green Chem, 2016, 18, 3582-3593.
- B. Winbal. Piracetam, A review of pharmacological properties and clinical uses. CNS Drug Rev. 2005, 11, 169-182.
- P. Singh, V. Dimitriou, R. P. Malajan, A.W. Crossley, Double-blind comparison between doxapram and pethidine in the treatment of postanaesthetic shivering. Br. J. Anaesth. 1993, 71, 685-688.
- M. S. F. Franco, G. A. Casagrande. C. Raminelli, S. Moura, M. Rossatto, F.H. Quina, L. Pizzuti, Ultrasound-Promoted Environmentally Friendly Synthesis of 5-(3,3,3- Trifluoro-2-oxopropylidene) pyrrolidin-2- ones, Synth. Commun, 2015, 45, 692-701.
- M. B-Brlek, M. Meanwel, R. Britton, Direct synthesis of imino-C-nucleoside analogues and other biologically active iminosugars, Nature Communications, 2015, 1-6
- S. Mukhopadhyay, S. Chandra Pan, Organocatalytic asymmetric synthesis of highly
substituted pyrrolidines bearing a stereogenic quaternary centre at the 3-position, Org. Biomol. Chem., 2018, 16, 9349-9353
- Q. Zhu, H. Jian, J. Li, Liu. Sh.Ch. XiaM. Zhang, Concise and Versatile Multicomponent Synthesis of Multisubstituted Polyfunctional Dihydropyrroles, J. Comb. Chem, 2009, 11, 685-696.
- A. Loupy, Microwaves in Organic Synthesis; Wiley-VCH, Weinheim; second Ed, 2006.
- P. Compain, V. Chagnault, O.R. Martin, Tactics and strategies for the synthesis of iminosugar C-glycosides: a review, Tetrahedron Asymmetry, 2009, 20, 672
- D. Declerck, S. Josse, A. Van. NhienNguen, D. Postel, Synthesis of 2-Aminocyclopropyl Pyrrolidines from Glycoaminonitriles, Tetrahedron. Lett, 2009, 2171-2173.
- R.D. Borcherdinf, A. S. Scholtz, T. R. Borchard. “Synthesis of analogues of neplanocin A: utilization of optically active
dihydroxycyclopentenones derived from carbohydrates, Journal of Organic Chemistry, 1987, 52, 5457-5461,
- X. Zhou, W.J. Liu, L. P. Ye J. Q. Huang, A versatile approach to pyrrolidine azasugars and homoazasugars based on a highly diastereoselective reductive benzyloxymethylation of protected tartarimide Tetrahedron, 63, 2007, 6346-6357.
- J.T. Pinhey, S. Sternhell, Aust. J. Chem., 1985, 38, 1155-1161.
DOI: http://dx.doi.org/10.13171/mjc8119021922cb
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
