The use of bentonite in heterogeneous medium as an efficient recyclable catalyst in the synthesis of iminoesters
Abstract
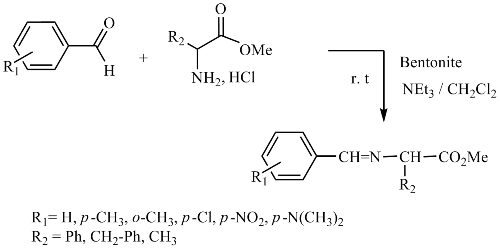
A new solid catalyzed synthesis of α-iminoesters is described. Various α-aminoesters react readily with aryl aldehydes to produce the corresponding imines in heterogeneous medium in the presence of bentonite (montmorillonite from oust region of Algeria). The products have been isolated in good to excellent yields.
This new environmentally, friendly catalyst provides significantly higher yields than traditional methods during relatively short reaction times for the preparation of the target compounds.
Full Text:
PDFReferences
- (a) C. NÃ jera, M. de Garcia Retamosa, J. M. Sansano, A. Cosar, F. P. Cossio, Tetrahedron Asymmetry, 2008, 19, 2913-2923.
(b) D. J. Ages, I. Parakash, D. R. Schaal, Chem. Rev., 1996, 96, 835-875.
(c) Wei Zhang, Chem. Rev., 2009, 109, 749-795.
(d) M. M. Rodriguez, C. NÃ jera, J. M. Sansano, F. L. Wu, Tetrahedron Asymmetry, 2010, 21, 1184-1186.
(e) V. A. Ioutsi, A. A. Zadorin, P. A. Khavrel, N. M. Belov, N. S. Ovchinnikova, A. A. Goryunkov, O. N. Kharybin, E. N. Nikolaev, M. A. Yurovskaya, L. N. Sidorov, Tetrahedron, 2010, 66, 3037-3041.
(f) A. Soret, C. Muller, R. Guillot, L. Blanco, S. Deloisy, Tetrahedron, 2011, 67, 698-705.
- L. Rao, Resonance, 2007, 12, No 8, 65-75.
- M. Abid, M. Savolainen, S. Landge, J. Hu, G. K. Surya Prakash, G. A. Olah, B. Török, Journal of Fluorine Chemistry, 2007, 128, 587–594.
- W. H. Pirkle, J. R. Hauske, J. Org. Chem., 1977, 42, 2436–2439. 5 - I. Moretti , G. Tone., Synthesis, 1970, 141.
- D. P. Roelofsen, H. Van Bekkum, Reel. Trav. Chim., 1972, 91, 605-610.
- G. Stork, A. Y. W. Leong, A. M. Touzin, J. Org. Chem., 1976, 41, 3491-3493.
- R. Grigg, H. Q. Nimal Gunaratne, J. Kemp, J. Chem. Soc. Perkin Trans I, 1984, 41.
- M. J. O’Donnel, J. M. Boniece, S. E. Earps, Tetrahedron Lett., 1978, 30, 2641-2644.
– J. H. Clark, Green Chem., 1999, 1, 1.
- (a) M. D. Bhor, N. S. Nandurkar, M. J. Bhanushali, B. M. Bhanage, Catal. Lett., 2006, 112, 45-50.
(b) M. D. Bhor, N. S. Nandurkar, M. J. Bhanushali, B. M. Bhanage, Ultrasonics Sonochemistry, 2008, 15, 195–202
(c) N. Lahbabi, Z. Rais, M. Hajjaji, S. Kcim, Afrique Science, 2009, 05, 14-24.
- M. P. Atkins, J. Williams, J. A. Ballantine, J. H. Purnell, Eur. Pat. Appl., 1988, EP 284397 A1. 13 - (a) S. Kleeman, M. Nygren, R. Wagner, Ger. Pat., DE, 1985, 3, 114-165.
(b) T. Vu Moc, H. Petit, P. Maitte, Bull. Soc. Chim. Belg., 1980, 89, 759-761
- P. Laszlo, J. Lucchetti, Tetrahedron Lett., 1984, 25, 2147.
– (a) D. Saib, A. Foucaud, J. Chem. Res. Synop., 1987, 372.
(b) G. D. Yadav, S. R. More, Applid Clay Science, 2011, doi: 10.1016/j.clay. 2011. 03.005.
- S. Hunig, K. Huber, E. Benzig, Chem. Ber., 1962, 95, 926.
- B. Labiad, D. Villemin, Synth. Commun., 1989, 19, 31.
B. Labiad, D. Villemin, Synthesis, 1989, 143.
- (a) J. A. Ballantine, J. H. Purnell, J. M. Thomas, Clay Miner., 1983, 18, 347.
(b) J. A. Ballantine, J. H. Purnell, J.M. Thomas, M. Davies, M. Rayanakorn, K. J. Williams, J. Mol. Catal., 1984, 26, 37.
(c) J. M. Adams, S. E. Davies, S. H. Graham, J. M. Tomas, J. Catal., 1982, 78, 197-208.
- D. U. Singh, S. D. Samant, J. Mol. Catal. A. Chem., 2004, 223, 111-116.
- M. J. O’Donnell, R. L. Polt, J. Org. Chem., 1982, 47, 2663-2665.
– (a) L. Duhamel, S. Fouquay, J. C. Plaquevent, Tet. Lett., 1986, 27, 4975-4978.
(b) S. G. Pyne, J. Safaei, A. K.Schafer, A. Javidan, B. W.Skelton, A. H. White, Aus. J. Chem., 1998, 51, 137-158.
(c) I. V. Saratovskikh, V. V. Kalashnikov, V. V Ragulin, Russ J Gen Chem., 1999, 69, 1173-1175.
(d) C. Najera, M. De Retamosa, J. M. Sansano, Org. Lett., 2007, 9, 4025-4028.
DOI: http://dx.doi.org/10.13171/mjc.1.1.2011.20.04.20
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
