Monoclinic Li2TiO3 Nano-particles via sol-gel method: Structure and impedance spectroscopy
Abstract
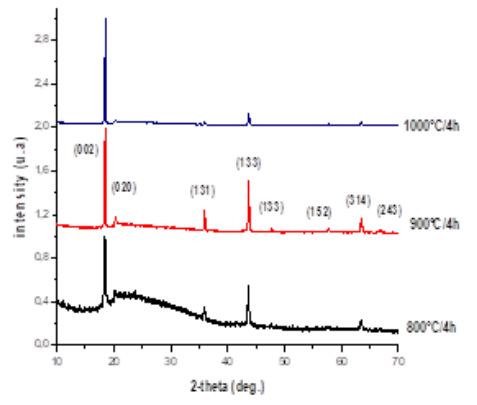
Pure phase Li2TiO3 nano-particles were synthesized by the sol-gel method, and the structural properties were examined with X-ray diffraction (XRD) technique. The latter showed that these materials, heat treated at relatively low temperature 900°C during 4h compared to the conventional solid-state reaction which calcination temperature is about 900–1100°C for 10 h; crystallize in the monoclinic phase without the presence of secondary phases. The microstructure of the LT ceramic (sintered at 1100°C) were determined by SEM, and good crystalline nature was observed with an average of granular size 2 μm. Moreover, the impedance spectroscopy showed at a higher temperature of 500°C the low-frequency arc due either to the grain boundary or sample-electrode charge transport processes.
Full Text:
PDFReferences
C. Johnson, E. Hollenberg, G. W. Roux and H. Watanabe , Current experimental activities for solid breeder development, Fusion Engineering and Design 1989, 8, 145-153.
L.Giancarli, V. Chuyanov, M. Abdou, M. Akiba, B. G. Hong, R. Lässe and Y. Strebkov, Test blanket modules in ITER: an overview of proposed designs and required DEMO-relevant materials. Journal of Nuclear Materials 2007, 367, 1271-1280.
N. Roux, J.Avon, A. Floreancing, J. Mougin, B. Rasneur, and S.Ravel, Low-temperature tritium releasing ceramics as potential materials for the ITER breeding blanket, Journal of nuclear materials 1996, 233, 1431-1435.
N.Roux, S.Tanaka, C. Johnson, and R. Verrall, Ceramic breeder material development, Fusion engineering and design 1998, 41(1), 31-38.
J. P. Kopasz, J. M. Miller and C. E. Johnson, Tritium release from lithium titanate, a low-activation tritium breeding material, Journal of nuclear materials 1994, 212, 927-931.
H. Kleykamp, Phase equilibria in the Li-Ti-O system and physical properties of Li2TiO3. Fusion Eng Des 2002, 61-62, 361-366.
G. Izquierdo, A.R. West, Mater. Res. Bull 1980, (15), 1655.
J.C. Mikkelsen, J. Am. Ceram. Soc 1980, (63), 331.
X. Wu, Z. Wen, B. Lin, X. Xu, Sol-gel synthesis and sintering of nanosize Li2TiO3 powder, Materials Letters 2008, 62 (6-7), 837-839.
C.H. Jung, J.Y. Park, S.J. Oh, H.K. Park, Y.S. Kim, D.K. Kim, J.H. Kim, Synthesis of Li2TiO3 ceramic breeder powders by the combustionprocess, Journal of Nuclear Materials 1998, 253, 203-212.
A. Sinha, S.R. Nair, P.K. Sinha, Single-step synthesis of Li2TiO3 powder, Journal of Nuclear Materials 2010, 399,162-166.
C.H. Jung, S.J. Lee, W.M. Kriven, J.Y. Park, W.S. Ryu, A polymer solution technique for the synthesis of nano-sized Li2TiO3 ceramic breeder powders, Journal of Nuclear Materials 2008, 373, 194-198.
M. Castellanos, A.R. West, Order-disorder phenomena in oxides with rock salt structure the system Li2TiO3-MgO, Journal of Materials Science 1979, 14, 450-454.
M.D. Aguas, G.C. Coombe, I.P. Parkin, New solid state routes to lithium transition metal oxides via reaction with lithium oxide, Polyhedron 1998, 17, 49-53.
G. Bhaskar Kumar, S. Buddhudu, Synthesis and emission analysis of RE3+ (Eu3+ or Dy3+): Li2TiO3 ceramics, Ceramics International 2009, 35, 521-525.
F. Krimech, S. Sayouri and T. Lamcharfi. Synthesis, structural and dielectric properties of Li-doped BaTiO3 nanopowders by sol-gel method, Journal of Ceramic Processing Research 2017, 18, 536-542.
D. Cruz, H. Pfeiffer, S. Bulbulian, Solid State Sci 2006, 8, 470.
H. Kleykamp, Fusion Eng. Des. 2002, 61-62, 361-366.
TA. Denisova, LG. Maksimova, EV. Polyakov, NA. Zhuravlev, SA.Kovyazina, ON. Leonidova, DF. Khabibulin, Yur'eva EI Metatitanic acid: synthesis and properties. Russ J Inorg Chem 2006, 51, 691-699.
R. Ramaraghavulu, S. Buddhudu, G. Bhaskar Kumar, Ceramics International 2011, 37, 1245-1249.
Th. Fehr, E. Schmidbauer. Electrical conductivity of Li2TiO3 ceramics, Solid State Ionics 2007, 178, 35-41.
DOI: http://dx.doi.org/10.13171/mjc8319051109fza
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
