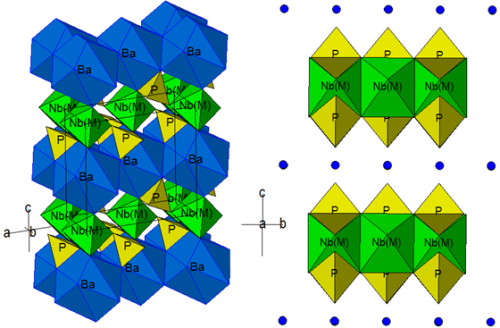
Structure, Infrared and Raman spectroscopic studies of the new Ba(NbV 0.5MIII0.5)(PO4)2 (MIII = Al, Cr, Fe, In) yavapaiite compounds ‘series
Abstract
Full Text:
PDFReferences
- N. Clavier, R. Podor, N. Dacheux, Crystal chemistry of the monazite structure, J. Eur. Ceram. Soc., 2011, 31, 941-976.
- J. J. Finney, N. Nagaraja Rao, The crystal structure of Chéralite, Am. Mineral., 1967, 52, 13-19.
- K. Fukuda, A. Moriyama, T. Iwata, Crystal structure, phase transition and anisotropic thermal expansion of barium zirconium diorthophosphate, BaZr(PO4)2, J. Solid State Chem., 2005, 178, 2144-2151.
- A. Leclaire, M. M. Barel, J. Chardon, B. Raveau, A Mo(IV) Monophosphate, BaMo(PO4)2, with the Yavapaiite Layer Structure, J. Solid State Chem., 1995, 116,
-368.
- K. Popa, G. Wallez, D. Bregiroux, P. Loiseau, MIIGe(PO4)2 (M=Ca, Sr, Ba) Crystal structure, phase transitions and thermal expansion, J. Solid State Chem., 2011, 184, 2629-2634.
- D. Zhao, H. Zhang, Z. Xie, W. L. Zhang, S. L. Yang, W. D. Cheng, Syntheses, crystal and electronic structures of compounds AM(PO4)2 (A = Sr, M = Ti, Sn; A = Ba, M = Sn), Dalton Trans., 2009, 27, 5310-5318.
- W. L. Zhang, C. S. Lin, Z. Z. He, H. Zhang,
Z. Z. Luo, W. D. Cheng, Syntheses of three members of A(II)M(IV)(PO4)2: luminescence properties of PbGe(PO4)2 and its Eu3+ doped powders, Cryst. Eng. Comm., 2013, 15, 7089-7094.
- G. Wallez, D. Bregiroux, K. Popa, P. E. Raison, C. Apostolidis, P. Lindqvist-Reis, R. J. M. Konings, A. F. Popa, BaAnIV(PO4)2 (AnIV = Th, Np) A New Family of Layered Double Phosphates, J. Inorg. Chem., 2011, 110-115.
- E. Morin, G. Wallez, S. Jaulmes, J. C. Couturier, M. Quarton, Structure of PbIISnIV(PO4)2: Stereochemical Activity of the Lead II Lone Pair, J. Solid State Chem., 1998, 137, 283-288.
- G. Blasse, G. J. Dirksen, The luminescence of Barium Titanium Phosphate BaTi(PO4)2, Chem. Phys. Lett., 1979, 62, 19-20.
- Z. J. Zhang, J. L. Yuan, X. J. Wang, D. B. Xiong, H. H. Chen, J. T. Zhao, Y. B. Fu, Z. M. Qi, G. B. Zhang, C. S. Shi, Luminescence properties of CaZr(PO4)2: RE (RE = Eu3+, Tb3+, Tm3+) under x-ray and VUV–UV excitation, Phys. D Appl. Phys., 2007, 40, 1910-1914.
- A. Aatiq, R. Hassine, R. Tigha, I. Saadoune, Structures of two newly synthesized A0.50SbFe(PO4)3 (A=Mn, Cd) Nasicon phases, Powder Diffr., 2005, 20, 33-39.
- A. Aatiq, R. Tigha, S. Benmokhtar, Structure, infrared and Raman spectroscopic studies of new Sr0.50SbFe (PO4)3 and SrSb0.50Fe1.50 (PO4)3 Nasicon phases, J. Mater. Sci., 2012, 47,
-1364.
- A. Aatiq, R. Tigha, R. Hassine, I. Saadoune, Crystallochemistry and structural studies of two newly CaSb0.50Fe1.50(PO4)3 and Ca0.50SbFe(PO4)3 Nasicon phases, Powder Diffr., 2006, 21, 45-51.
- A. Aatiq, My R. Tigha, R. Fakhreddine,
A. Marchoud, Structure and spectroscopic characterization of the two PbSb0.5Fe1.5(PO4)3 and Pb0.5SbFe(PO4)3 phosphates with Nasicon type-structure, J. Mater. Environ. Sci., 2015, 6, 3483-3490.
- A. Aatiq, A. Marchoud, H. Bellefquih, My
R. Tigha, Structural and Raman spectroscopic studies of the two M0.50SbFe(PO4)3 (M = Mg, Ni) NASICON phases Powder Diffr., 2017, 32, 40-51.
- A. Aatiq, My R. Tigha, R. Fakhreddine,
D. Bregiroux, G. Wallez, Structure, infrared and Raman spectroscopic studies of newly synthetic AII(SbV0.50FeIII0.50)(PO4)2 (A=Ba, Sr, Pb) phosphates with yavapaiite structure Solid State Sci., 2016, 58, 44-54.
- J. RodrÃguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction Physica B. Condensed Matter., 1993, 192, 55-69.
- B. Srinivasulu, M. Vithal, Preparation of a new family of NASICON type phosphates Ca0.5NbMP3O12 (M = Fe, Al, Ga and In) and characterization of the iron systems by Mossbauer spectroscopy Mater. Sci. Lett., 1999, 18, 1771-1773.
- A. Le Bail, H. Duroy, J. L. Fourquet, Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction, Mater. Res. Bull., 1988, 23, 447-452.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides Acta Crystallographica Section A, 1988, 32,
-767.
- D. Zhao, Fa-X. Ma, H. Yang, W. Wei, Y.-C. Fan, L. Zhang, X. Xin, Structure twinning, electronic and photoluminescence properties of yavapaiite-type orthophosphate BaTi(PO4)2,
J. Phys. Chem. Solids, 2016, 99, 59–65
- I. D. Brown, D. Altermatt, Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database Acta Cryst. Section B, 1985, 41, 244-247.
- M. Th. Paques-Ledent, AIIBIV(XO4)2 Phosphates and Arsenates with Yavapaiite structure I: isostructural relationship and vibrational study, Inorg. Nucl. Chem., 1977, 39, 11-17.
- A. Ann McConnel, J. S. Aderson, C. N. R. Rao, Raman spectra of niobium oxides, A Mol. Biomol. Spectrosc., 1976, 32, 1067-1076.
- A. El Jazouli, C. Parent, J. M. Dance, G. Le Flem, P. Hagenmuller, J. C. Viala, Na4Nb(PO4)3, a material with a reversible crystal-glass transformation: Structural and optical comparison, J. Solid State Chem., 1988, 74, 377-384.
- F. D. Hardcastle, I. E. Wachs, Determination of niobium-oxygen bond distances and bond orders by Raman spectroscopy, Solid State Ion., 1991, 45, 201-213.
- L. Popović, D. de Waal, J. C. A. Boeyens, Correlation between Raman wavenumbers and P—O bond lengths in crystalline inorganic phosphates, J. Raman Spectrosc., 2005, 36,
-11.
- W. G. Fateley, F. R. Dollish, N. T. McDevitt, F.F. Bentley, Infrared and Raman selection rules for molecular and lattice vibrations the correlation method, Wiley-Interscience, New York, 1972.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A, Fifth ed, Wiley–Interscience, New York 1997.
- G. Butt, N. Sammes, G. Tompsett, A. Smirnova, O. Yamamoto, Raman spectroscopy of superionic Ti-doped Li3Fe2(PO4)3 and LiNiPO4 structures, J. Power sources, 2004, 134, 72-79.
DOI: http://dx.doi.org/10.13171/mjc851907094raf
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
