A study of olive mill waste water removal by a biosorbent prepared by olive stones
Abstract
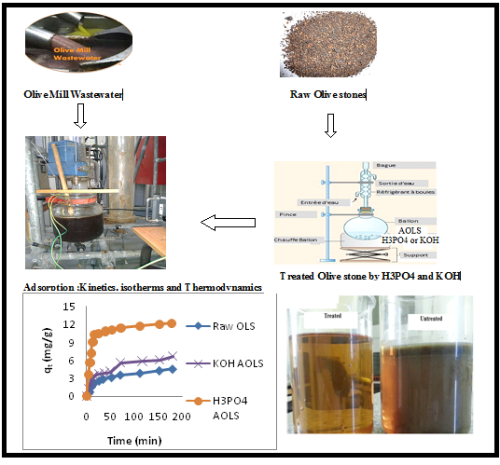
In this work, olive mill wastewater (OMW) such as dyes is very toxic even present as traces in industrial wastewater effluents. It may constitute a potential pollution source of ground waters and hence it has to be eliminated. Various low cost adsorbents have been studied for their applicability in treatment of different types of effluents. In this study, the potential of activated carbon derived from Olive Stones was studied for the removal of OMW. The biosorption of OMW from aqueous solutions by Olive Stones (OLS) as a low-cost, natural and eco-friendly biosorbent was investigated and by KOH and H3PO4 treated Olive Stones (AOLS). Biosorption kinetic data were properly fitted with the pseudo-second-order kinetic model. The experimental isotherm data were analyzed using Langmuir, Freundlich isotherm equations. The best fit was obtained by the Langmuir model with a Langmuir maximum monolayer biosorption capacity of 189,83 mg/g for OMW. The biosorption was exothermic in nature (H° = -105,54 kJ/mol). The reaction was accompanied by a decrease in entropy. The Gibbs energy (G°) increased when the temperature was increased from 303 to 320 °K indicating a decrease in feasibility of biosorption at higher temperatures. The results have established good potentiality for the Olive Stones to be used as a sorbent for the removal of olive mill wastewater.
Full Text:
PDFReferences
- D. Simeonov, L. Spasov, P. Simeonova, Statistical calibration of model solution of analytes. Ecol. Chem. Eng. S., 2012, 19, 67-75.
- A. Mittal, L. Kurup, V.K. Gupta, Use of waste materials-bottom ash and de-oiled soya, as potential adsorbents for the removal of amaranth from aqueous solutions. J. Hazard. Mater. 2005, 117, 171-178.
- A.Y. Dursun, O. Tepe, Internal mass transfer effect on biodegradation of phenol by Ca-alginate immobilized Ralstonia eutropha. J. Hard. Mater. 2005, 126, 105-111.
- A. Dąbrowski, P. Podkościelny, Z. Hubicki, M. Barczak, Adsorption of phenolic compounds by activated carbon—a critical
- B. Taraba, Adsorption heats of phenol on activated carbon using adapted method of immersion calorimetry. Journal of thermal analysis and calorimetry, 2011,107, 923-926.
- S. Wang, H. Niu, T. Zeng, X. Ma, Y. Cai,
X. Zhao, Fabrication of magnetic mesoporous carbon and its application for adsorptive removal of 2, 4, 6-trichlorophenol (TCP) from aqueous solution. Cryst. Eng. Comm. 2014, 25, 5598-5607.
- A. Dąbrowski, P. Podkościelny, Z. Hubicki,
M. Barczak, Adsorption of phenolic compounds by activated carbon-a critical review. Chemosphere, 2005, 58,1049-1070.
- Y. Wang, J. Niu, Y. Li, T. Zheng, Y. Xu,
Y. Liu, Performance and mechanisms for removal of perfluorooctanoate (PFOA) from aqueous solution by activated carbon fiber. RSC Advances, 2015, 106, 86927-86933.
- L. A. Rodrigues, T.M.B. Campos, M.O. Alvarez-Mendes, A. dos Reis Coutinho, K.K. Sakane, G.P. Thim, Phenol removal from aqueous solution by carbon xerogel. Journal of sol-gel science and technology, 2012, 63,
-210.
- S. Yao, H. Lai, Z. Shi, Biosorption of methyl blue onto tartaric acid modified wheat bran from aqueous solution. Iranian journal of environmental health science & engineering, 2012, 9,16.
- M., Seredych, T.J. Bandosz, Removal of cationic and ionic dyes on industrial− municipal sludge based composite adsorbents. Industrial & engineering chemistry research, 2007, 46, 1786-1793.
- M. Ahmaruzzaman, S.L. Gayatri, Activated tea waste as a potential low-cost adsorbent for the removal of p-nitrophenol from wastewater. Journal of Chemical & Engineering Data, 2010, 5511, 4614-4623.
- H. Mine Kurtbay, Z. Bekçi, M. Merdivan,
K. Yurdakoç, Reduction of ochratoxin A levels in red wine by bentonite, modified bentonites, and chitosan. Journal of agricultural and food chemistry, 2008, 56, 2541-2545.
- C. Namasivayam, S. Sumithra, Adsorptive removal of catechol on waste Fe (III)/Cr (III) hydroxide: equilibrium and kinetics study. Industrial & engineering chemistry research, 2004, 43, 7581-7587.
- O. Zahraa, L. Sauvanaud, G. Hamard,
M. Bouchy, Kinetics of atrazine degradation by photocatalytic process in aqueous solution. International Journal of Photoenergy, 2003, 5, 87-93. â
- Lin SH, Juang RS. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J. Envion. Manage. 2009, 90, 1336-1349.
- G. Dursun, H. Cicek, A.Y. Dursun, Adsorption of phenol from aqueous solution by using carbonised beet pulp. Journal of hazardous materials, 2005,125, 175-182.
- B. Özkaya, Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. Journal of hazardous materials, 2006,129, 158-163.
- P.A. Mangrulkar, S.P. Kamble, J. Meshram, S.S. Rayalu, Adsorption of phenol and o-chlorophenol by mesoporous MCM-41. Journal of Hazardous Materials, 2008, 160, 414-421.
- X. Jin, M.Q. Jiang, X.Q. Shan, Z.G. Pei,
Z. Chen, Adsorption of methylene blue and orange II onto unmodified and surfactant-modified zeolite. J. Colloid Interface Sci. 2008, 328, 243-247.
- M. Iqbal, M.Z. Ahmad, I.A. Bhatti, K. Qureshi, A. Khan, Cytotoxicity reduction of wastewater treated by advanced oxidation process. Chemistry International, 2015,1, 53-59.
- B. Dass, P. Jha, Batch adsorption of phenol by improved activated Acacia nilotica branches char: equilibrium, kinetic and thermodynamic studies. Int J Chem Tech Res, 2015, 8, 269-279.
- M. Niaounakis, C.P. Halvadakis, Olive processing waste management: literature review and patent survey, Elsevier, 2006, 5.
- A. Nait-Merzoug, A. Benjaballah, O. Guellati, Préparation et caractérisation d'un charbon actif à base d'un déchet agricole, 2016.
- N. F. Cardoso, R.B. Pinto, E.C. Lima,
T. Calvete, C.V. Amavisca, B. Royer, I.S. Pinto, Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination, 2011, 269, 92-103.
- D.J. Cao, X. Yang, G. Geng, X.C. Wan, R.X. Ma, Q. Zhang, Y.G. Liang, Absorption and subcellular distribution of cadmium in tea plant (Camellia sinensis cv. 'œShuchazao'. Environmental Science and Pollution Research, 2018, 25, 15357-15367.
- L.B. Lim, N. Priyantha, D.T.B. Tennakoon, H.I. Chieng, M.K. Dahri, M. Suklueng, Breadnut peel as a highly effective low-cost biosorbent for methylene blue: equilibrium, thermodynamic and kinetic studies. Arab J Chem. 2017, doi: 10. S3216-S3228.
- E. Ayranci, O. Duman, Adsorption behaviors of some phenolic compounds onto high specific area activated carbon cloth. Journal of hazardous materials, 2005, 124, 125-132.
- O. Moradi, M. Yari, P. Moaveni, M. Norouzi, Removal of p-nitrophenol and naphthalene from petrochemical wastewater using SWCNTs and SWCNT-COOH surfaces. Fullerenes, Nanotubes and Carbon Nanostructures, 2012, 20, 85-98.
- L. Seid, D. Chouder, N. Maouche, I. Bakas,
N. Barka, Removal of Cd (II) and Co (II) ions from aqueous solutions by polypyrrole particles: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2014, 45, 2969-2974.
- M. Arami, N.Y. Limaee, N.M. Mahmoodi, Evaluation of the adsorption kinetics and equilibrium for the potential removal of acid dyes using a biosorbent. Chem. Eng. J. 2008,139, 2-10.
- R.J. Umpleby II, S.C. Baxter, M. Bode, J.K. Berch Jr, R.N. Shah, K.D. Shimizu, Application of the Freundlich adsorption isotherm in the characterization of molecularly imprinted polymers. Analytica Chimica Acta, 2001, 435, 35-42.
- N. Barka, A. Assabbane, A. Nounah, L. Laanab, Y.A. Ichou, Removal of textile dyes from aqueous solutions by natural phosphate as a new adsorbent. Desalin. 2009, 235, 264-275.
- A. Gürses, A. Hassani, M. Kıranşan, Ö. Açışlı, S. Karaca, Removal of methylene blue from aqueous solution using by untreated lignite as
potential low-cost adsorbent: kinetic, thermodynamic and equilibrium approach. J. Water Process Eng. 2014, 2, 10-21.
- S. Arellano-Cárdenas, T. Gallardo-Velázquez, G. Osorio-Revilla, M. López-Cortéz, B. Gómez-Perea, Adsorption of phenol and dichlorophenols from aqueous solutions by porous clay heterostructure (PCH). J. Mex.
Chem. Soc. 2005, 49, 287-291.
- J.P. Silva, S. Sousa, J. Rodrigues, H. Antunes, J.J. Porter, I. Gonçalves, S. Ferreira-Dias, Adsorption of acid orange 7 dye in aqueous
solutions by spent brewery grains. Sep. Purif. Technol. 2004, 40, 309-315.
- A. Alatrache, A. Cortyl, P. Arnoux, M.N. Pons, O. Zahraa, Sulfamethoxazole removal from polluted water by immobilized photocatalysis. Toxicological & Environmental Chemistry, 2015, 97, 32-42.
- S.A. Mohtashami, N.A. Kolur, T. Kaghazchi,
R. Asadi-Kesheh, M. Soleimani, Optimization of sugarcane bagasse activation to achieve adsorbent with high affinity towards phenol. Turkish Journal of Chemistry, 2018, 42, 1720-1735
- M. Ahmaruzzaman, S.L. Gayatri, Activated neem leaf: a novel adsorbent for the removal of phenol, 4-nitrophenol, and 4-chlorophenol from aqueous solutions. Journal of Chemical & Engineering Data, 2011,56, 3004-3016.
- O. Tepe, A.Y. Dursun, Combined effects of external mass transfer and biodegradation rates on removal of phenol by immobilized Ralstonia eutropha in a packed bed reactor. Journal of hazardous materials, 2008,151, 9-16.
- P.D. Pathak, S.A. Mandavgane, B.D. Kulkarni, Fruit peel waste as a novel low-cost bio adsorbent. Reviews in Chemical Engineering, 2015, 31, 361-381.
- W. Wei, Q. Wang, A. Li, J. Yang, F. Ma, S. Pi, D. Wu, Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Scientific reports, 2016, 6, 31575.
- A.T.M. Din, B.H. Hameed, A.L. Ahmad, Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Hazard. Mater.2009,161,1522-1529.
- A.H. Mahvi, A. Maleki, A. Eslami, Potential of rice husk and rice husk ash for phenol removal in aqueous systems. American Journal of Applied Sciences. 2004, 321-326.
- J. A. Hefne, W.K. Mekhemer, N.M. Al, O.A. Aldayel, T. Alajyan, Kinetic and thermodynamic study of the adsorption of Pb (II) from aqueous solution to the natural and treated bentonite. International Journal of Physical Sciences, 2008, 3, 281-288.
DOI: http://dx.doi.org/10.13171/mjc851907138wy
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
