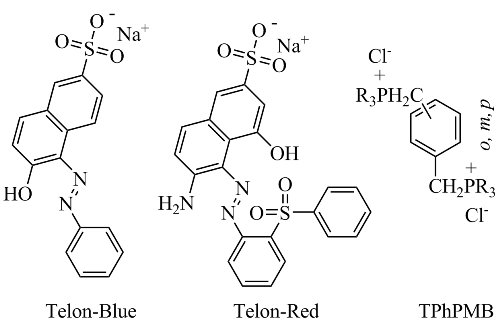
Telon dye removal from Cu(II)-containing aqueous media using p-diphosphonium organo-montmorillonite
Abstract
Full Text:
PDFReferences
- E. Assaad, A. Azzouz, D. Nistor, A. V. Ursu, T. Sajin, D. Miron, F. Monette, P. Niquette, R. Hausler, Appl. Clay Sci., 2007, 37, 258–274.
- J. Y. Bottero, K. Kathib, Water Res., 1994, 28, 483-490.
- S. A. Boyd, Environ. Sci. Technol., 1996, 9, 24-28.
- S. A. Boyd, G. Sheng, B. J. Trappen, Environ. Sci. Technol., 2001, 35, 4227-4234.
- D. C. Rodriguez- Sarmiento, J. A. Pinzon-Bello, Appl. Clay Sci., 2001, 18, 173-181.
- M. K. Sang, J. B. Dixon, Appl. Clay Sci., 2001, 18, 111-112.
- M. A. N. Lawrence, R. K. Kukkadpu, S. A. Boyd, Appl. Clay Sci., 1998, 13, 13-20.
- M. A. Didi, B. Makhoukhi, A. Azzouz, D. Villemin, Appl. Clay Sci., 2009, 42, 336-344.
- B. Makhoukhi, M. A. Didi, D. Villemin, A. Azzouz, Grasas Aceites, 2009, 60, 343-349.
- B. Makhoukhi, M. A. Didi, H., Moulessehoul, A. Azzouz, D. Villemin, Appl. Clay Sci., 2010, 50, 354-361.
- N. Al-Bastaki, F. Banat, Resour. Conserv. Recycling, 2004, 41, 103-114.
- S. A. Boyd, W. F. Jaynes, Appl. Clay Sci., 1993, 1-4, 17-30.
- A. S. Ozcan, B. Erdem, J. Hazard. Mater., 2005, 135, 141-148.
- A. S. Ozcan, J. Hazard. Mater., 2006, 142, 165-174.
- Y. H. Shen, Chemosphere, 2001, 44, 989–995.
- G. Sheng, S. Xu, S. A. Boyd, Water Res., 1996, 30, 1483–1489.
- M. Wieczorek, A. Krysztafkiewicz, T. Jesionowski, J. Phys. Chem. Solids, 2004, 65, 447–452.
- M. Arroyo, M. Suarez, M. Lopez-Manchado, J. Fernandez, J. Nanosci. Nanotechnol., 2006, 6, 2151–2154.
- A. Hartwig, D. Putz, M. Schartel, M. Wendschuh-Josties, Macromol. Chem. Phys., 2003, 204, 2247–2257.
- W. Xie, R. Xie, W. Pan, D. Hunter, B. Koene, Chem. Mater., 2002, 14, 4837–4845.
-International Union of Pure and Applied Chemistry (IUPAC), 1997. Compendium of Chemical Terminology, 2nd ed. Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford, 1997.
- R. D. Lillie, P. Pizzolato, P. T. Donaldson, Histochemistry. 1976, 49, 23-35.
-T. Bechtold, R. Mussak, Handbook of Natural Colorants. John Wiley and Son, Chichester (UK), 2009, pp.337.
- B. Tabak, S. F. Afsin, H. Icbudak, J. Therm. Anal. Calorim., 2005, 81, 311–314.
- J. Y. Yoo, J. Choi, T. Leer, J.W. Park, Water Air Soil Poll., 2003, 154, 225-237.
- M. Crocker, R. M. Herold, J. Mol. Catal., 1991, 70, 209-216.
- R. Margalef, P. Salagre, E. Fernandez, C. Claver, Catal. Lett., 1999, 60, 121–123.
- J. W. Park, P. Jaffe, Environ. Sci. Technol., 1993, 27, 2559–2565.
- R. Gullick, W. Weber, Environ. Sci. Technol., 2001, 35, 1523–1530.
- B. Makhoukhi, M. A. Didi, D. Villemin, Mater. Lett., 2008, 62, 2493–2496.
-A. Azzouz, Interactions Chitosane – particules colloidales: Synergie avec les argiles. Chapter 12. In:Crini, G., Badot, P. M., Guibal, E. (Eds). Chitine et Chitosane – Du biopolymère à l’application: Presses Universitaire de Franche-Comté, Université de Franche-Comté, Besançon, France, 2009, pp. 231-255.
- A. Azzouz, Chitosan and derivatives– behavior in dispersed state and applications. In: Davis, S. P. (Ed). Chitosan: Manufacture, Properties, and Usage. Series: Biotechnology in Agriculture, Industry and Medicine, Publisher: Nova-Publishers, New-York, USA, 2010, 4th quarter.
https://www.novapublishers.com/catalog /product_info.php?products_id=12712.
- Y. S. Ho, D. A. J. Wase, C. F. Forster, Environ. Technol., 1996, 17, 71-77.
- A. S. Özcan, A. Özcan, J. Colloid Interf. Sci., 2004, 276, 39–46.35 - A. K. Bajpai, L. Rai, Indian J. Chem. Techn., 2010, 17, 17-27.
- N. Kannan, M. M. Sundaram, Dyes Pigments, 2001, 51, 25-40.
- W. J. Weber, J. C. Morris, J. Sanitary Eng. Div. Am. Soc. Civil Eng., 1963, 89, 31–39.
- A. Mastalir, Z. Kiraly, G. Szollosi, M. Bartoky, J. Catal., 2000, 194, 146–152.
- H. A. Patel, R. S. Somani, H. C. Bajaj, R.V. Jasra, Curr. Sci. India, 2007, 92, 1004–1009.
- J. Baham, G. Sposito, J. Environ. Qua., 1994, 23, 147–153.
-G. Grigoropoulou, P. Stathi, M. A. Karakassides, M. Louloudi, Y. Eligiannakis, Colloid Surface A, 2008, 320, 25-35.
- S. J. Anderson, G. Sposito, Soil Sci. Soc. Am. J., 1991, 55,1569–1576.
-R. A. Schoonheydt, C. T. Johnston, Surface and Interface Chemistry of Clay Minerals, in: Bergaya, F., Lagaly, B. K. J., G. (Eds), Handbook of Clay Science – I. Developments in Clay Science. Elsevier, Amsterdam, 2006, pp. 87-113.
- M. B. McBride, Environmental Chemistry of Soils, Second Ed., Oxford University Press, 1994, pp. 352.
-A. Azzouz, D. Messad, D. Nistor, C. Catrinescu, A. Zvolinschi, S. Asaftei, Appl. Catal. A-Gen., 2003, 241, 1-13.
-A. Azzouz, D. Nistor, D. Miron, A. V. Ursu, T. Sajin, F. Monette, P. Niquette, R. Hausler, Thermochim. Acta, 2006, 449, 27-34.
DOI: http://dx.doi.org/10.13171/mjc.1.2.2011.18.10.19
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Mediterranean Journal of Chemistry
