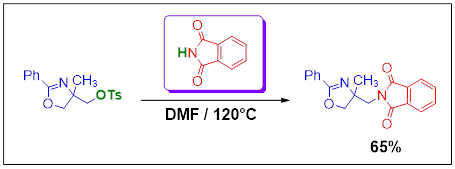
Synthesis, Crystal Structure and IR Spectrum studies of 2-(4-Methyl-2-phenyl-4,5-dihydro-oxazol-4-ylmethyl)- isoindole-1,3-dione
Abstract
The organo-amino compound of title 2-(4-methyl-2-phenyl-4,5-dihydro-oxazol-4-ylmethyl)-isoindole-1,3-dione was synthesized by the mixture of (4-methyl-2-phenyl-4,5-dihydrooxazol-4-yl)methyl-4-methylbenzenesulfonate and isoindoline-1,3-dione in N,N-dimethylformamide with a yield of around 65%. The structural study of the compound, C19H16N2O3, is realized using single crystal X-Ray diffraction which shows that this compound crystallizes in the monoclinic system (P21/c, Z = 4) with the unit cell parameters: a = 14.3728 (13) Å, b = 9.6829 (10) Å, c = 11.8964 (12) Å and β = 107.384 (3). The refinement of the structure by the least-squares method with complete matrix leads to the following reliability factors R/Rw are 0.044/0.130.
In the crystal, the molecules are linked together by hydrogen bonds and π…π interactions.
The Infrared spectroscopic studies show the bands confirming the presence of the groups C=O, C-O, C-N, -CH3, -CH2 and =CH.
Full Text:
PDFReferences
- Sharma, P.K. A review: Antimicrobial agents based on nitrogen and sulfur-containing heterocycles. Asian. J. Pharm. Clin. Res. 2017, 10, 47.
- Bandgar, B.P.; Patil, S.A.; Gacche, R.N.; Korbad, B.L.; Hote, B.S.; Kinkar, S.N.; Jalde, S.S. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730. DOI: 10.1016/j.bmcl.2009.11.068.
- Ruddarraju, R.R.; Murugulla, A.C.; Kotla, R.; Tirumalasetty, M.C.B.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R. Design, synthesis, anticancer activity and docking studies of the ophylline containing 1,2,3-triazoles with variant amide derivatives. Med. Chem. Commun. 2016, 8, 176. DOI:10.1039/C6MD00479B.
- Khanage, S.G.; Raju, A.; Mohite, P.B.; Pandhare, R.B. Analgesic Activity of Some 1,2,4-triazole Heterocycles Clubbed with Pyrazole, Tetrazole, Isoxazole and Pyrimidine. Adv. Pharm. Bull. 2013, 3, 13. DOI:10.5681/apb.2013.003.
- Fernandes, G.F.S.; Chin, C.M.; Santos, J.L. Advances in Drug Discovery of New Antitubercular Multidrug-Resistant Compounds. Pharmaceuticals 2017, 10, 51. DOI:10.3390/ph10020051.
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in synthetic approach to and antifungal activity oftriazoles. Beilstein J. Org. Chem. 2011, 7, 668. DOI:10.3762/bjoc.7.79.
- Zhang, M.; Chen, Q.; Yang, G. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421. DOI:10.1016/j.ejmech.2014.10.065.
- Bhat, K.S.; Poojary, B.; Prasad, D.J.; Naik, P.; Holla, B.S. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009, 44, 5066. DOI:10.1016/j.ejmech.2009.09.010.
- Mekni, N.; Bakloiti, A. Synthesis of new 1-substituted 4-perfluoroalkyl tetrazol-5-ones. J. Fluorine Chem. 2008, 129, 1073. DOI:10.1016/j.jfluchem.2008.06.019.
- Lim, S.J.; Sunohara, Y.; Matsumoto, H. Action of fentrazamide on protein metabolism and cell division in plants. J. Pestic. Sci. 2007, 32, 249. DOI:10.1584/jpestics.G07-07.
- Myznikov, L.V.; Hrabalek, A.; Koldobskii, G.I. Drugs in tetrazole series. Chem. Het, Compounds 2007, 43, 1.
- Modarresi Alam, A.R.; Nasrollahzadeh, M. Synthesis of 5-arylamino-1H (2H)-tetrazoles and 5-amino-1-aryl-1H-tetrazoles from secondary arylcyanamides in glacial acetic acid: A Simple and Efficient Method. Turk J. Chem. 2009, 267, 33. DOI:10.3906/kim-0808-44.
- Hiriyanna, S.G.; Basavaiah, K.; Dhayanithi, V.; Bindu, A.; Sudhaker, P.; Pati, H.N. Simultaneous Determination of Several Angiotensin-II-Receptor Antagonists by Liquid Chromatography. Anal Chem. Indian J. 2008, 7, 568.
- Katritzky, A.R.; Jain, R.; Petrukhin, R.; Denisenko, S.; Schelenz, T. QSAR correlations of the algistatic activity of 5-amino-1-aryl-1H-tetrazoles. SAR QSAR Environ. Res. 2001, 12, 259. DOI:10.1080/10629360108032915.
- Butter, R.N. Comprehensive heterocyclic chemistry, Katritzky A. R., Rees, C. W. Eds.; Pergamon Press: New York, NY, USA. 1984; Vol. 5: Part 4A, 791.
- Eddy, N.O.; Ita, B.I.; Ibisi, N.E.; Ebenso, E.E. Experimental and Quantum Chemical Studies on the Corrosion Inhibition Potentials of 2-(2-Oxoindolin-3-Ylideneamino) Acetic Acid and Indoline-2,3-Dione. Int. J. Electrochem. Sci. 2011, 6, 1027.
- Mihit, M.; Salghi, R.; El Issami; S.; Bazzi, L.; Hammouti, B.; Ait Addi, E.; Kertit, S. A study of tetrazoles derivatives as corrosion inhibitors of copper in nitric acid. Pigm. Resin Technol. 2006, 35, 151. DOI:10.1108/03699420610665184.
- Mihit, M.; El Issami, S.; Bouklah, M.; Bazzi, L.; Hammouti, B.; Ait Addi, E.; Salghi, R.; Kertit, S. The inhibited effect of some tetrazolic compounds towards the corrosion of brass in nitric acid solution. Appl. Surf. Sci. 2006, 252, 2389. DOI: 10.1016/j.apsusc.2005.04.009.
- Dafali, A.; Hammouti, B.; Aouniti, A.; Mokhlisse, R.; Kertit, S.; Elkacemi, K. 2-Mercapto-1-methylimidazole as corrosion inhibitor of copper in aerated 3% NaCl solution. Ann. chim. Sci. Mat. 2000, 25, 437. DOI:10.1016/S0151-9107(00)80019-7.
- Larabi, L.; Benali, O.; Mekelleche, S.M.; Harek, Y. 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl. Surf. Sci. 2006, 253, 1371. DOI:10.1016/j.apsusc.2006.02.013.
- Li,W.; He, Q.; Pei, C.; Hou, B. Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim Acta. 2007, 52, 6386. DOI:10.1016/j.electacta.2007.04.077.
- Scendo, M.; Hepel, M. Inhibiting properties of benzimidazole films for Cu(II)/Cu(I) reduction in chloride media studied by RDE and EQCN techniques. Corros. Sci. 2007, 49, 3381. DOI:10.1016/j.corsci.2007.03.022.
- Zarrouk, A.; Hammouti, B.; Zarrok, H.; Bouachrine, M.; Khaled, K.F.; Al-Deyab, S.S. Corrosion Inhibition of Copper in Nitric Acid Solutions Using a New Triazole Derivative. Int. J. Electrochem. Sci. 2012, 7, 89.
- Zerga, B.; Attayibat, A.; Sfaira, M.; Taleb, M.; Hammouti, B.; EbnTouhami, M.; Radi, S.; Rais, Z. Effect of some tripodal bipyrazolic compounds on C38 steel corrosion in hydrochloric acid solution. J. Appl. Electrochem. 2010, 40, 1575.
- Zerga, B.; Saddik, R.; Hammouti, B.; Taleb, M.; Sfaira, M.; EbnTouhami, M.; Al-Deyab, S.S.; Benchat, N. Effect of New Synthesised Pyridazine Derivatives on the Electrochemical Behaviour of Mild Steel in 1M HCl Solution: Part-1. Int. J. Electrochem. Sci. 2012, 7, 631.
- Zerga, B.; Hammouti, B.; EbnTouhami, M.; Touir, R.; Taleb, M.; Sfaira, M.; Bennajeh, M.; Forsal, I. Comparative inhibition study of new synthesized pyridazine derivatives towards mild steel corrosion in hydrochloric acid. Part-II: Thermodynamic proprieties. Int. J. Electrochem. Sci. 2012, 7, 471.
- Huang, H.-M.; Gao, J.-R.; Hou, L.-F.; Jia, J.-H.; Han, L.; Ye, Q.; Li, Y.-J. The first iodine improved 1,3-dipolar cycloaddition: facile and novel synthesis of 2-substituted benzo[f]isoindole-4,9-diones. Tetrahedron 2013, 69, 9033. DOI:10.1016/j.tet.2013.08.029.
- Arjunan, V.; Saravanan, I.; Ravindran, P.; Mohan, S. Structural, vibrational and DFT studies on 2-chloro-1H-isoindole-1,3(2H)-dione and 2-methyl-1H-isoindole-1,3(2H)-dione. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2009, 74, 642. DOI:10.1016/j.saa.2009.07.012.
- Yue, H.; Lu, F.; Shen, C.; Quan; J.-M. Structure-based design of benzo[e]isoindole-1,3-dione derivatives as selective GSK-3β inhibitors to activate Wnt/β-catenin pathway. Bioorganic Chemistry 2015, 61, 21. DOI:10.1016/j.bioorg.2015.05.009.
- Atmani, A.; El Hallaoui, A.; El Hajji, S.; Roumestant, M.L.; Viallefont, P. (1991). From Oxazolines to Precursors of Aminoacids. Synthetic Communications 1991, 21, 2383. DOI:10.1080/00397919108021599.
- Bruker (2016). APEX3 (Version 5.054), SAINT+ (Version 6.36A), SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. SHELXT-2014 program (2015a). Acta Cryst. A71, 3-8. DOI: 10.1107/S2053273314026370
- Sheldrick, G. M. SHELXL-2018 program (2015b). Acta Cryst. C71, 3-8. DOI: 10.1107/S2053229614024218.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. SADAPS (2015). J. Appl. Cryst. 48, 3-10. DOI: 10.1107/S1600576714022985.
- Farrugia, L. J. ORTEP 3 program (2012). J. Appl. Cryst. 45, 849-854. DOI: 10.1107/S0021889812029111.
DOI: http://dx.doi.org/10.13171/mjc92190916245aa
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Mediterranean Journal of Chemistry
